So in this article, we are going to look into the history and mystery of Belousov–Zhabotinsky reaction (in short BZ reaction).
Let us start with my meme :P
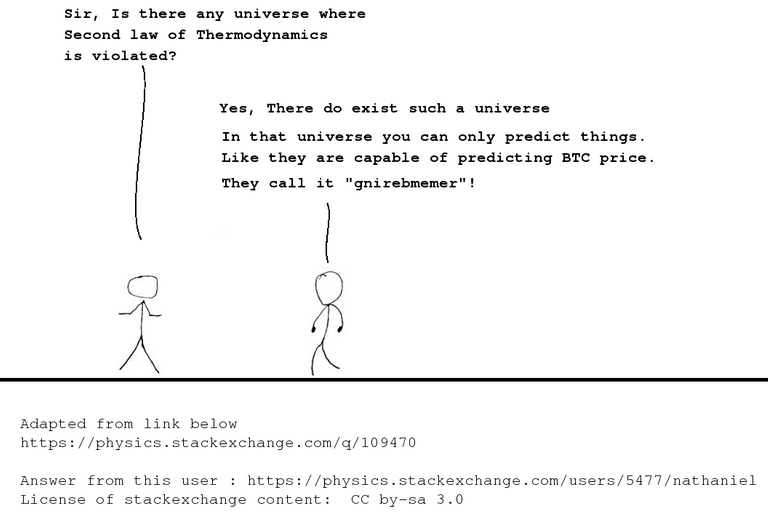
( The above meme is adapted from the content here. This was an answer from a user named nathaniel in physics.SE. License of SE content: CC-by-SA ver 3.0 )
Let us start with this youtube video which demonstrates some chemical reaction:
As you will notice that the liquid inside the jar oscillates between different colors back and forth. There are different variants of these kinds of reactions. It was initially observed by a Soviet chemist/biophysicist called Boris Belousov. The original reaction is said to be very complicated.
From Wiki:
The mechanism for this reaction is very complex and is thought to involve around 18 different steps which have been the subject of a number of research papers.
So I am not going to explain the chemical reaction here and that is not the focus here. But in case you want to see the reaction, please visit this Wiki page. You will the details there.

Boris Belousov ( Image Source: Wiki )
HISTORY
Belousov was trying to see if he could find any non-organic analogs of Krebs cycle. Krebs cycle in short in a series of biochemical reaction cycle which happens inside aerobic organisms. He was finally able to do so and the reactions exhibited oscillations as in the above video. When he tried to publish his findings in journals, all of them rejected it. And the reason was that most chemists at those times never believed that oscillations can occur in chemical reactions. Oscillations were considered as a violation of Second law of thermodynamics, which is considered very fundamental. Some even doubted that Belousov's findings were fake. After a lot of struggle, Belousov finally published his results in an obscure journal. And that was in 1959.[1] But it remained unknown to the outside world.
A very brief explanation of Second Law of Thermodynamics
It basically says that the entropy on average in a closed system increases. As an example, consider a hypothetical closed box with some gas particles in it. The Second law says that the gas particles would scatter out in space rather than aggregate with other gas particles and restrict itself to a very small region (purely based on the statistical reasons).
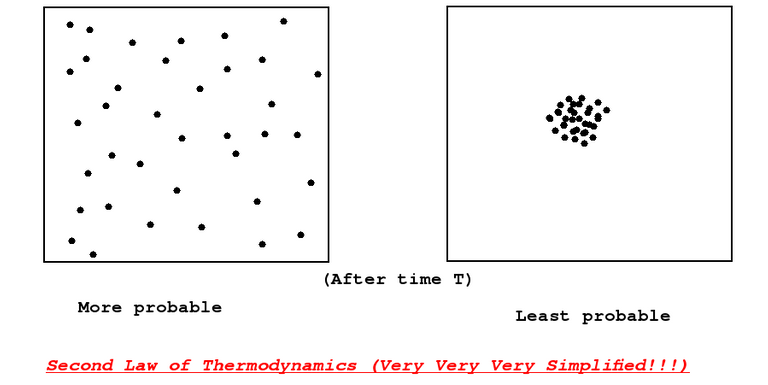
The second law of thermodynamics indirectly says that there is an irreversibility in the direction of time. Entropy is this arrow of time.
MYSTERY
The mystery as mentioned before in these reactions where oscillations, which made most chemists think that second law is violated here. Since the Second law is so fundamental and cannot be violated, the only reason was to disregard Belousov's findings. But there was a catch here. The catch was at different levels:
- First of all the reactions(and thus oscillations) never lasted forever, although it was very long in timescale. If you want this reaction to go forever you should supply the chemicals periodically.
- Secondly, you need to stir it continuously to reactions to proceed. (So there is an external source of energy here)
- Thirdly, in those times chemists where thinking mostly reactions in terms of a 2 state process. They never thought chemical reactions having many intermediate states.
Let us take 4 cases as in below:
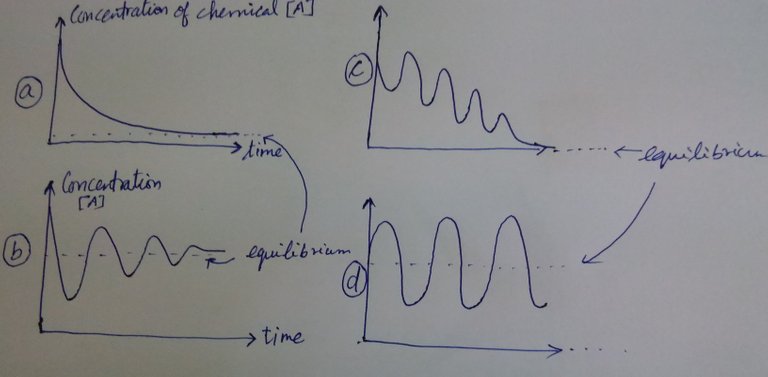
The 4 plots show that concentration of chemical species [A] fluctuating with time. In the case (a), you see that that [A] decays to an equilibrium state. Fairly simple, right? Nothing fancy. It is like 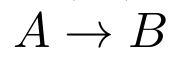 . So [A] decays. In (b), (c) and (d) you see oscillations. So the question is whether they violate second law?
. So [A] decays. In (b), (c) and (d) you see oscillations. So the question is whether they violate second law?
In the case (b) and (c), you see that there is a reduction in the intensity of oscillations and they arrive at an equilibrium state finally. So it is possible. Case (d) should be dealt with care. If in the system (d), the oscillations were arising without supplying external chemical species and applying external energy, then that is a violation of Second Law. But that is not what Belusouv did. There was no faking nor Second law was violated.
Back to HISTORY and how the MYSTERY "got solved"
But there were some scientists who were really fascinated by the oscillations they saw in nature and biology. For example, we have something called circadian rythm in our bodies, which is an internal clock which is related to day-night cycle. So it was clear that oscillations do exist in nature. Still, they don't violate the second law because these systems are not closed in nature. They are open systems. They exchange energy and matter with outside world.
Anatol Zhabotinsky who came know about the work of Belousov from his supervisor and started working on this problem. And he managed to prove the theory to outside world. It was also understood by now that these class of reactions can be modeled as Reaction-Diffusion systems. [I have few reaction-diffusion articles on my blog. You can access it here: 1, 2]
Anyway, Belousov died in 1970 without getting any recognition. In 1980, the Soviet government awarded Lenin prize to this legendary scientist(posthumously) along with Zhabotinsky and others.
Finally one more youtube video of BZ reactions, this time in petri-dish. The perturbations or instabilities spatially will cause spiral waves and concentric circular waves in these systems. It is known that these are non-linear dynamical systems which exhibits chaos. More about those technical terms later.
I will stop here by paying my respects to Belousov.
References
[1] : http://campus.usal.es/~licesio/Biofisica/Winfree_JCE1984.pdf
Hi,
@dexterdev here
Please Upvote and resteem my posts if you like my content.
I would like to hear your feedback. So please do comment those.
In case you have questions, please shoot those in comments.
All images without image sources are my creations :)

gif courtesy: @rocking-dave
Great article! Never knew such beautiful reaction can exists! Is it the oscillation is caused by redox reaction? I remember back in school Cu2+ is blue Cu3+ is green? I might be wrong lol. But awesome work man.
The explanation for oscillation is not very easy actually. But to understand it in simple and enternaining way I have 2 videos foryou: 1 and 2. These are each 4 minutes long and simplified. IF you have any specific doubts, please do ask. It would be nice if you watch these videos. I have not focused on the chemistry itself in my article. The focus was to tell that there is no violation of 2nd law and this oscillation happens because of the intermediary states in the energy landscape. :) Yes the original reaction contains one electron redox catalyst. Ref