SIMPLE SCIENCE


What about beryllium?

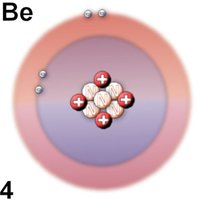
What's up, hivers. Little by little we are advancing within the periodic table knowing the characteristics and uses of the elements, today we will talk about Beryllium. It is the fourth element of the periodic table, belonging to the so-called alkaline earth metals, it has certain characteristics that make it a widely used element in the metallurgical, aeronautical, aerospace and pharmaceutical industries. Beryllium has several properties, one of them is that in spite of its low weight it has a high density, it has a grayish metallic color and sometimes it is confused with aluminum. As we can see, as we advance in the study of the periodic table, an electron, a proton and a neutron are added to the previous element, resulting in the next element. This is a very scarce element in our planet and the countries that have reserves in exploitation are: United States, Russia, China and Brazil, where the United States is the main exporter of this metal.[1-2]

What is Beryllium used for?
Beryllium is a compound that is not found in nature in a pure state, it is rather extracted in the form of other compounds such as chrysoberyl. This metal is widely used in the metallurgical industry to create alloys with copper, which are very hard alloys and low weight, become up to 4 times more resistant than normal copper in addition to being light, making it a widely used alloy in the aviation industry where it is used in the construction of aircraft parts, as they are good at resisting metal fatigue. In addition, it is used in the elaboration of tools, since they do not produce sparks and are widely used in industries where flammable materials are handled. Since it is a metal with a high melting point, since it is alloyed with other metals, it can be used in the manufacture of satellites and airplanes, since it resists great weights, loads and temperatures. [2-3]

Despite its 12 known isotopes, Beryllium is considered monostopic
Beryllium has 12 known isotopes of which only one is stable, thus making this element one of the monostopic group. Be-9, is the most stable isotope known, unlike its other isotopes that have a half-life of seconds, it has 5 neutrons. Among the most stable known isotopes are Be-10 with a half-life of 1.6 million years and Be-7 with a half-life of 53 days. Why is Be-9 considered the most stable isotope? Well, because it does not produce radioisotope traces, like Be-10 or Be-7. [3-4]
Curiosities about Beryllium.
Beryllium has many shapes and styles depending on its alloy or reaction with other compounds for example: there is the common Beryllium that is cloudy and opaque, there is the Emerald Beryllium, which is a green like grass and is represented as such when creating alloys with chromium and vanadium, we also find the Aquamarine Beryllium, created by the alloy with Iron, this being of a blue-green color and many more like the noble Beryllium, etc.[6]
Other uses of Beryllium.
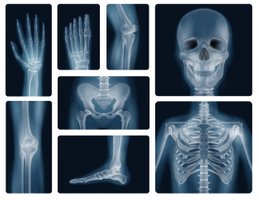
Among other uses of Beryllium in x-ray analysis, due to its density it becomes almost transparent to the passage of x-ray radiation, making it a good material for this type of analysis, where small sheets of Beryllium are used to carry out the procedure and thus obtain a better image. [2-3]
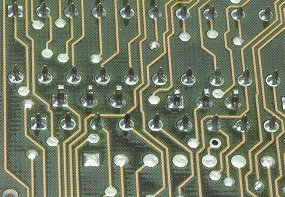
In addition, Beryllium is used in electronics, as an important material in the manufacture of speakers, solder points in some electrical circuits, given its characteristics that make it a very resistant material. [3]
In the reference area I will leave you more information to enrich your reading.

Disturbing Note on Beryllium.
Beryllium salts and improper exposure to this metal, by inhalation can cause damage to the lungs and even create cancer, acute chemical pneumonitis is caused by exposure to beryllium.
Recommendations.
Beryllium is not a highly dangerous material, but it is advisable to be very careful with its salts and always maintain a use of the compound under strict safety standards.

to carry out the translation into English you need the support of two translators for complex words or correct grammar: DeepL Traslate | Dictionry cambridge
