SIMPLE SCIENCE


What's wrong with Lithium?

Lithium is the third element of the periodic table, this element was discovered in 1817 and has in its nucleus 3 protons and 3 neutrons. This is the first element found in alkaline metals, it is white, shiny and is one of the softest metals in nature. It has the lowest melting point of alkaline metals and also oxidizes very easily in the presence of air or water. This element is mainly used in the metalworking industry and in the manufacture of batteries, not for nothing they are called lithium batteries. If you want to know more about this element, stay in this post, because I will talk about many of the uses and properties of this interesting chemical element.[1]

What is Lithium used for?
Lithium is mainly used in the metallurgic industry. It is one of the active compounds for purification and elimination of impurities in the refining of metals such as iron, nickel, copper and zinc and their alloys. Lithium is very reactive in the removal of oxygen, nitrogen, sulfur, carbon, hydrogen and sulfur. Another very important use of lithium is energy storage batteries, as its name indicates, is used in the manufacture of lithium batteries, being these high power, which were designed mainly for electric cars, these widely used for the manufacture of rechargeable batteries that are used in cell phones, cameras and a large number of electrical devices. [1-2]

Did you know that Lithium has eleven isotopes?
We already know that the isotopes of an element are given by the variation in the amount of neutrons in its nucleus and Lithium has a total of 11 different isotopes, surprisingly stable but with a half-life of only a few micro or nano seconds, but there are two very stable lithium isotopes that represent the highest percentage in abundance which is Li-6 (Lithium-6) and Li-7 (Lithium-7). Li-7 consists of 3 protons, 4 neutrons and 3 electrons, this is the most abundant in nature and is considered one of the fundamental elements created in the Big Bag and this represents approximately 92.5%. One of the uses of this Li-7 isotope is in lithium-7 hydroxide in the alkalinization of the coolant in pressurized water reactors, unlike Li-6 which is the result of the impoverishment of Li-7 resulting in only 7.5% in abundance, this isotope is used when isolated and placed in hydrogen pumps.[3-4]
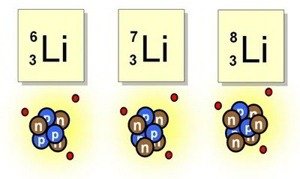
Curiosities of Lithium.
In recent years lithium batteries are being developed that are more durable, as many of the batteries used today have had storage problems, discharging very quickly. The development of a new generation of batteries that can store up to five times more charge is very close to becoming a reality, where prototypes have been developed that were originally called lithium-ion, renamed Lithium-air because when these batteries are discharged they release oxygen. [5]
Other uses of Lithium.
Among other uses of lithium is in the organic synthesis, to make reactions in the determination of metals in the laboratory as well as it is made at industrial level, given the capacity that this element has in the elimination of non metals elements. There are also lithium compounds used in the treatment of people with bipolar disorders and depression, controlling patients with psychopathological conditions, these salts are lithium carbonate (Li2CO3) and lithium citrate. [2-3]

It is also used in the manufacture of synthetic rubber, participating in the initial phase of polymerization. Lithium definitely has an incredible variety of uses ranging from the metallurgical to the pharmaceutical industry.[3]

In the reference area I will leave you more information to enrich your reading.

Disturbing note on Lithium.
This is a highly reactive element with water when it is in its pure state, in short, most of the elements belonging to the alkaline group have this property.
Recommendations.
lithium is an element that must be handled with care, given its high reactivity with water when it is in its pure state; likewise the supply of drugs containing lithium since they have a strong reaction in the brain.

| Sources | Link |
|---|---|
| 1 | Chemical properties of Lithium |
| 2 | Lithium, History and Applications |
| 3 | Lithium chemical element |
| 4 | Lithium Isotopes |
| 5 | New lithium battery |
| 6 | lithium characteristics |
to carry out the translation into English you need the support of two translators for complex words or correct grammar: DeepL Traslate | Dictionry cambridge
