Ethanol, also known as ethyl alcohol, besides being produced with the purpose of obtaining alcoholic beverages (perhaps its best known use) or as antiseptic alcohol in the pharmaceutical sector, is an important raw material in organic synthesis processes in the chemical industry, obtaining important products derived from this compound, such as ethyl acetate and diethyl ether, widely used in the production of solvents, paints, plastics and other very valuable products for our modern life.
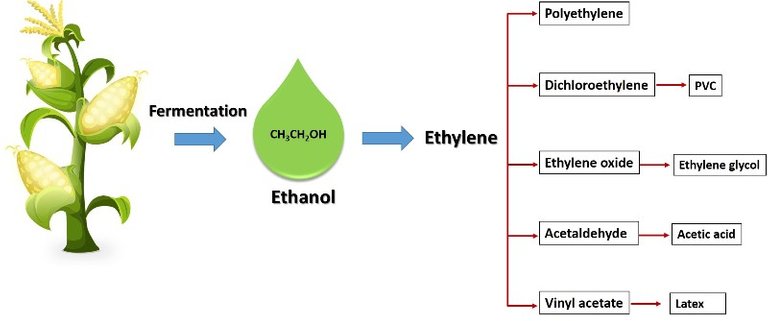
Bioethanol can be used as a raw material for other products. Source: @emiliomoron, contains public domain image.
Although ethanol is mainly produced by the petrochemical route, due to the pollution problems generated by this route, its production has been implemented through the fermentative transformation of biomass from renewable natural resources; we know that by fermenting a plant material rich in sugars, alcohol is obtained. Although this ethanol obtained from renewable sources has been mainly used as biofuel, it has the potential to be used to obtain other products for the chemical industry, since it has the same characteristics as ethanol obtained from petroleum.
But to obtain other products from ethanol, it is first required to convert it into ethylene through the catalytic dehydration process; ethylene is then used as an intermediate product for the synthesis of other compounds, such as polyethylene, ethylene oxide and acetaldehyde, among others. Therefore, very effective catalysts are needed to obtain a good yield of the reaction, but in most cases these catalysts are composed of noble metal oxides, which makes them very expensive.
The best option among the noble metals is the use of copper, but so far the best option among them that gives good yields and selectivity has not been found. That is why chemists from RUDN University proposed new catalysts based on aluminum and zirconium, modified with copper, the results were presented in the journal Catalysis Today.
Since it is necessary to improve the performance of catalysts using copper to ensure both high conversion and selectivity of the reaction, i.e. to favor maximum alcohol conversion and at the same time obtain the desired substances. To achieve this, the researchers combined two approaches; first, they combined oxides of various metals in nanocomposites: aluminum, cerium and zirconium, and synthesized five types of powders with different ratios of oxides at various temperature conditions, which allowed them to obtain different structures in the materials. The second step was to add copper. All the powders obtained previously were treated with an aqueous solution of copper nitrate and were reduced with hydrogen flow at 400°C.
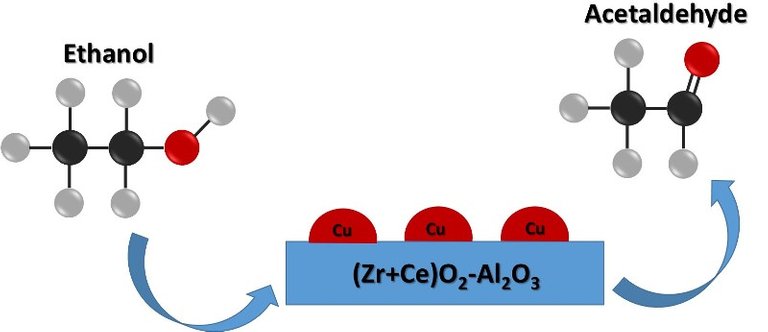
Ethanol conversion over the synthesized catalyst. Source: @emiliomoron.
The catalysts obtained based on (Zr+Ce)O2-Al2O3 modified with Cu were evaluated in the ethanol vapor dehydrogenation reaction. All the catalysts obtained showed high alcohol conversion and selectivity to acetaldehyde.
These results are very encouraging and represent a very important step in the development of alternative technologies for the manufacture of valuable products for our modern society, by means of chemical synthesis from vegetable raw materials using more efficient and economical catalysts, reducing the dependence on petroleum to obtain these products.
Thanks for coming by to read friends, I hope you liked the information. See you next time.
So what were these results that were very encouraging? Did it reduce costs? Did it hasten the production process? Compared to the plain copper, how efficient were the aluminum or zirconium combination exceeded the baseline?
Greetings friend. The research only involved the synthesis and catalytic evaluation of the solids obtained in laboratory conditions, being the initial phase of research on catalysts, so let's wait for the scale tests to make a comparison with other systems in terms of costs and other aspects. I say that they are encouraging since we can assume that the synthesized catalyst is more economical due to the use of copper instead of another noble metal, such as Pt or Pd, and because it showed a high conversion and selectivity, higher than 80%; so we can continue investigating this system to expand the information.
They used the sol-gel method. You don't use solids there. And ethanol is in liquid form as far as I know.
The graphical abstract on your source already mentions the answer "Up to 86% conversion with 97% selectivity". And it's not just plain Copper used but "oxide-derived" Copper compounds, that's why Aluminum and Zirconium were mentioned. I think you also may be interested in this Ethanol from Copper Catalyst too.