The transfer of electrons is facilitated by light acting on molecules or a group of molecules capable of undergoing oxidation-reduction reactions. Silver halides, particularly silver bromide, break down when exposed to light in such a way that the silver ion, which has been reduced to the atom state, gains an electron from the anion (halogen).
The photographic process:
The photographic process is based on the formation of tiny metallic silver crystallization centers within the emulsion during a brief illumination; during development of the exposed film, the process of reduction develops around these centers.

Pellicule- Source: Pixabay
Light-stripped electrons from bromine ions are transferred to the conduction band in silver bromide crystals where they are trapped by impurities or flaws in the crystal lattice. The silver ions diffuse in the direction of these traps, capturing the electrons and converting them into atoms. Then, a latent image develops. If we use an infrared source to illuminate an exposed bromide, the latent image will be lost because the infrared radiation's energy is only sufficient to remove an electron from a silver atom; after doing so, the electrons are thrown back into the conduction band and picked up by the bromine atoms. Conversely, the low energy of the infrared quanta is insufficient to remove an electron from a bromine ion.
A sensitizing effect is seen when a dye is present; the dye molecule (A) absorbs a luminous quantum, becomes excited, and then interacts with a silver ion to change it into an atom:
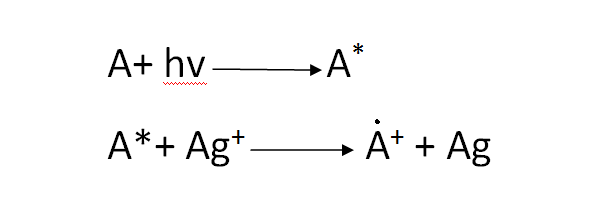
[Created by using microsoft paint and word]
The dye changes to the state of an ionic radical and further decomposes.
Photooxidation:
The creation of peroxide and hydroperoxide molecules results from the photooxidation of hydrocarbons by oxygen; cyclic hydrocarbons are particularly easily photooxidized; for instance, dimethylcyclohexene produces hydrocarbons:
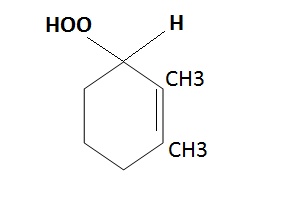
[Created by using microsoft paint]
It is worth noting that certain bodies with conjugated double bonds (such as chlorophyll) are capable of transmitting oxygen to the bodies to be oxidized when exposed to light. The phenomenon serves as the foundation for chemical theories of photosensitization. Schonberg believes that conjugated bonds are transformed into biradicals by the action of light:
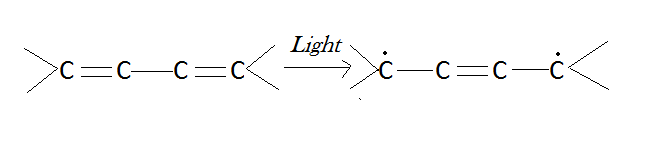
[Created by using microsoft paint]
Following oxygen fixing, these radicals become complex radicals:

[Created by using microsoft paint]
This final radical is responsible for oxidizing the oxidizable molecule that captures the oxygen atoms. Certain hydrocarbons can also act as oxygen transmitters; for example, rubrene fixes oxygen during photooxidation, producing a photooxide that decomposes on heating, releasing the fixed oxygen.
Other reactions, such as the fixation of quinones, alcohols, and ammonia on double bonds, among others, are facilitated by light radiation. The radicals created under the influence of light are likely to play a major role in all reactions:
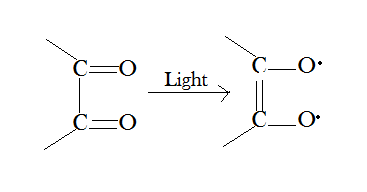
[Created by using microsoft paint]
References:
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
[Principles & Applications of Photochemistry, Brian Wardle,Wiley, ISBN 0470014938]
[R. OUAHES et B. DEVALLEZ- Chimie générale- Office des publacations universitaires- Alger]
[Norman S. Allen- Photochemistry and Photophysics of Polymeric Materials]
T. Mill- Reactions and Processes: Chemical and Photo Oxidation
Nice one. Light plays a very crucial role in facilitating many chemical reactions. It's more like a catalyst that aids many chemical reaction. This reaction is very important especially the photosynthesis is Agriculture which is very much important to man's survival
I agree with you, thanks for reading :)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Cool post my bro. I have always thought it was a cool pricess of photo too. Nice science lesson.
!LOL
Thank you :) have a nice day!
lolztoken.com
Her height is perfect.
Credit: reddit
@benainouna, I sent you an $LOLZ on behalf of @captainquack22
The LOLZ Tribe is here! Stake your LOLZ now to earn curation rewards and continue using the !LOLZ command. Please read our latest update for more information.
(4/6)