Atomic hydrogen forms an unstable solid hydride when it interacts directly with phosphorus, sulfur, arsenic, and even mercury. Atomic hydrogen converts the oxides of copper, lead, bismuth, and silver to metallic states at room temperature. It forms hydrogen peroxide when combined with oxygen at low temperatures.
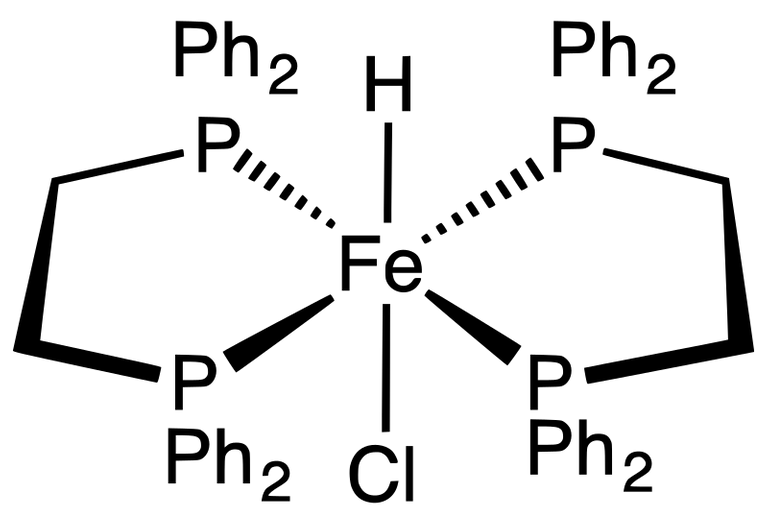
One of the easiest to obtain transition metal hydrides is HFeCl(dppe)2- Source: Wikipedia
Organic molecules respond to hydrogen atoms in a very specific way. In some cases (such as oleic acid), hydrogen atoms attach to double bonds to cause hydridation, while in other instances, atomic hydrogen rips another hydrogen atom from the molecule to create an H2 molecule. The recombinations of atoms, which evolve at such a rapid rate that practically an atom of hydrogen can only exist in the free state for about half a second, show this tendency to form molecules in a particularly marked way. The interesting thing about hydrogen recombination is that it can only take place if the energy released by the collision of two hydrogen atoms is transferred to a third atom, to any other particle, or to the container wall that the gas is being held in, the excess energy that has built up in the newly formed H2 molecule causes it to instantly dissociate into atoms, returning to its initial state if there is no material body to which it could transfer its excess energy. When a solid wall is in contact with the recombination, the heat that is released during the recombination is collected by the wall, which then becomes extremely hot (this is the Langmuir atomic burner principle). Atomic hydrogen stubbornly holds onto its single electron, requiring 315 kcal/atom-g of energy to dissociate it; energy is released in large amounts during the reverse reaction, which occurs when an electron attaches to a hydrogen ion. By absorbing a quantum of radiation's energy, electrons can become detached. The opposite process, which releases a tremendous amount of heat, most likely takes place on a large scale in areas of the cosmos with large stellar masses.
- Since 103 kcal/atom-g are released during the formation of molecules from atoms, it is not surprising that hydrogen exists in its least active and most stable form as the molecule H2.
Bibliographic references:
[General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
[Book- Chimie moderne- L.Nikolaiv]
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
this is the first post of yours I've seen, and I love it lol I'll definitely be sticking around to read more
!lolz
!pizza
lolztoken.com
He tells her about his job.
Credit: marshmellowman
@benainouna, I sent you an $LOLZ on behalf of @vagabond42069
Are You Ready for some $FUN? Learn about LOLZ's new FUN tribe!
(4/8)