Nitrogen and hydrogen combine to form ammonia under difficult circumstances:
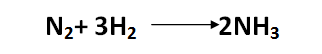
[Created by using word microsoft]
Ammonia is now the primary raw material used in the production of fertilizers and explosives, which has resulted in a significant revolution in industrial chemistry. Before 1917, Chilean nitrate was practically the primary source of nitrogenous acid, the majority of which was utilized in the production of explosives. The German scientist Haber deserves credit for this synthesis because he examined the previous equilibrium and the factors that affected it and discovered that by compressing the reactant gaseous mixture (300–1000 atm) and heating it to a medium temperature (400–500 °C), along with the use of a medium to speed up the reaction, this reaction can be accomplished with a good yield.
At high temperatures, hydrogen also combines with carbon to form methane, which constitutes the main part of natural gas released from oil wells:
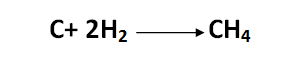
[Created by using word microsoft]
The significance of this gas is due to the intense heat it produces when burned with oxygen:
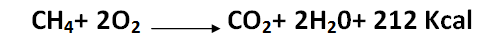
[Created by using word microsoft]
In addition, hydrogen can combine with group I and group II metals, with the exception of magnesium and beryllium, to create crystalline compounds that have an ionic structure and contain hydrogen as a negative ion. For instance, the reaction with sodium occurs when hydrogen is added to liquid metal at 320 °C:
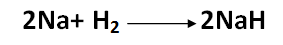
[Created by using word microsoft]
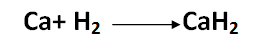
Also, the reaction with calcium requires heat:
[Created by using word microsoft]
The optical spectrum of hydrogen:
This emission spectrum is the most basic. It is produced experimentally by an electric discharge in a hydrogen-filled tube operating at low pressure (1 mmHg). Through this discharge, the molecules are split apart, and hydrogen atoms are excited. Electromagnetic radiation is emitted as the atoms transition from their various excited states back to their lower energy states.

Visible lines in the hydrogen emission spectrum- Wikipedia
The analysis of the radiation reveals that it is composed of four distinct light lines in each of the colors red, blue, indigo, and violet, which are designated as: Hα, Hβ, Hγ et Hδ respectively. It is the hydrogen atom's visible line spectrum.
- It is referred to as being in the ground state when the electron of the hydrogen atom is not excited and is in a low energy orbit. And this atom is said to be in an excited state when it is excited by a potential, for instance, when it absorbs energy that causes an electronic transition from the fundamental level to a higher energy permitted level.
- When an excited atom's electron enters an unstable state, it returns to a lower energy level and emits radiation. According to the hydrogen line spectrum, the electron's energy levels are quantized, meaning that only specific atomic energy levels are permitted.
Bibliographic references:
[General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
[Book- Chimie moderne- L.Nikolaiv]
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.