Other unstable atoms besides positronium are also known, like muonium, which is made up of an electron and a positive muon.
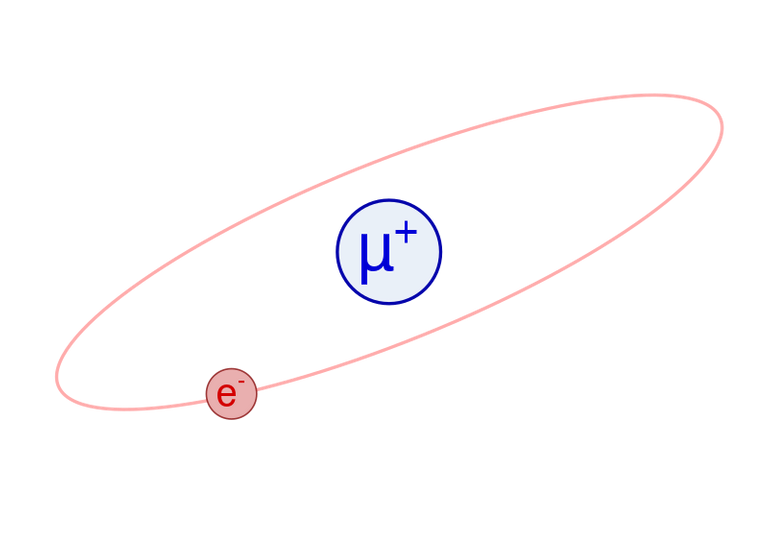
A muonium atom
Important inferences about polymerization, the distribution of electrical density in various complex compounds, the peculiarities of the electronic orbitals of metal ions, the mechanisms of oxidation-reduction reactions, the role of diffusion phenomena in chemical kinetics, etc. can be made using measurements of the probability of the appearance of positronium and the rate at which these unstable atoms disappear in their collisions with other particles.
It is useful to contrast the characteristics of positronium and hydrogen. The two forms of positonium, ortho and para, are similar to those of hydrogen. Orthopositonium is the more stable of the two, with a lifetime that ranges from 10 to the power minus 7 seconds in a gaseous medium to 10 to the power minus 9 seconds in the condensed phase. When attempting to research something, like the evolution of fast reactions, the lifetime of positronium is precisely what is measured. Similar to how hydrogen participates in reactions, positronium can also participate in those where chlorine and iron are involved.
- The lower mass, lower molecule binding energy (0.11 eV for Ps2 versus 4.5 eV for H2), and lower ionization potential of positronium make it distinct from hydrogen.
The production of dihydrogen:
Any element must always be differentiated between industrial preparation and preparation done in a laboratory. In the laboratory, simplicity of preparation is crucial, whereas cost is the primary consideration in industry.
- Preparation in the laboratory:
A- Diluted acids' effects on a variety of metals, including zinc, iron, magnesium, and tin:
Zn + 2HCl==== H2 + ZnCl2
In these reactions, alkaline and alkaline earth metals are not suitable because it is difficult to control the reaction because of how strongly hydrogen is released. Other metals, such as copper, silver, gold, mercury, and others, do not react with diluted acids to release hydrogen because their reduction potentials are above hydrogen's in the reduction potentials table.
Bibliographic references:
[General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
[Book- Chimie moderne- L.Nikolaiv]
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
[Livre- Chimie génerale- R.Ouahes]
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.