
Author: @madridbg, via Power Point 2010, using public domain images. flickr
Greetings and welcome dear readers of this prestigious platform, especially to all those users who make life in the community of greater scientific relevance of the #Hive platform, referring to @StemSocial.
In this sense, through the following lines of writing, we will analyze in a descriptive way the diverse syntheses or theses that are assumed at scientific level and that allow to establish the coexistence of similar molecular substances, which differ specifically due to the length of bond that conforms it.

INTRODUCTION

On our planet, we can visualize the existence of various types of substances that have different physical and chemical properties and determinant for the identification of the same and according to these properties, are the visible characteristics of each substance that we know and that we can determine according to the changes of state of matter.
Therefore, it is not surprising that we can find substances in solid, liquid or gaseous state and this behavior is due to the presence of existing intermolecular forces, which are responsible for keeping the molecular species related.
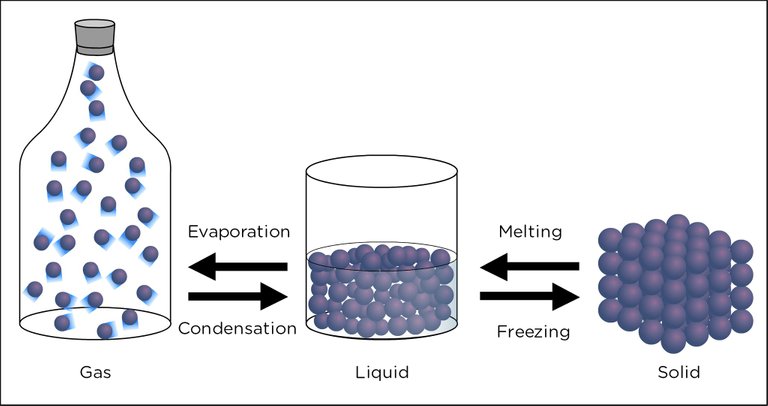
Fig. 2. Representation of the states of matter. Author: flickr
In such a way that the substances on our planet can pass from one state to another, i.e., they can solidify, condense, evaporate, among others. Therefore, and based on the above, it is assumed that the intermolecular forces have electrostatic characteristics, influenced by the interactions between the dipoles that make up the molecules, behavior that in scientific jargon is known as Van Der Waals forces.
Therefore, the objective of this research is to make known the intermolecular forces present in the twin chemical substances, as well as to validate according to scientific justification the veracity and existence of this type of molecules.

FUNCTIONALITY OF INTERMOLECULAR FORCES IN RELATION TO LONDON DISPERSION FORCE

Previously, we have determined that intermolecular forces are those that allow a direct correlation to be established between the molecules that make up a chemical substance, so it is not surprising that almost all substances with covalent bonds are composed of independent molecular units.
On the contrary, if only intramolecular forces existed, there would be no attraction between the surrounding molecules and consequently all the substances that are covalently bonded would be gaseous at any temperature. Based on this premise, it is assumed that beyond the intramolecular forces there must exist intermolecular forces that allow molecular attraction and consequently generate dipoles, which chemically are called London dispersion forces.
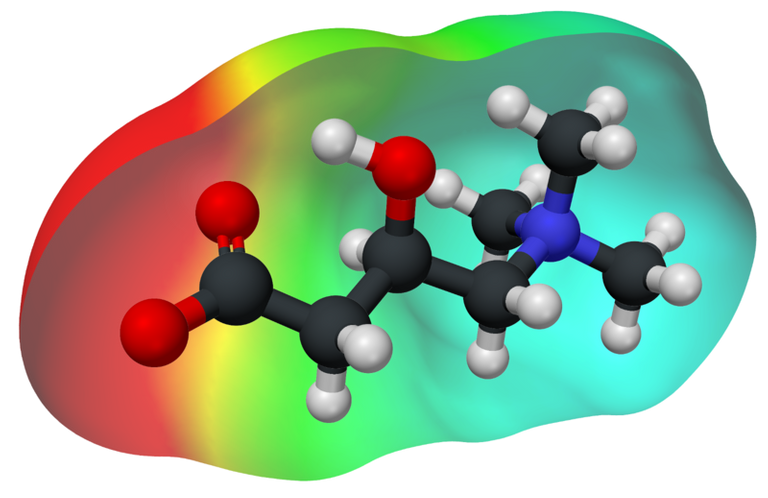
Fig. 3. Electron density of L-cannitine. Author: Manuel Almagro Rivas
In this way, the dispersion forces depend on the number of electrons in the atom and the relationship between them, under this approach it is determined how easy it is to polarize the electron density and consequently obtain a higher dispersion force.
In turn, the resulting intensity is what determines the melting and boiling points of a substance, hence the more intense the intermolecular forces, the higher the melting and boiling points of the compounds.
Let's analyze this behavior in depth, chemically we have heard that atoms are formed by electrons, protons and neutrons, so let's focus our attention on the first ones, which as a result of the continuous movement generate at the nucleus level a heterogeneous distribution of atomic charges, imagine a soccer field, when a team attacks, the players repel each other and therefore only in ephemeral moments the players are concentrated in one place of the field.
At this point, we say that the electrons have produced instantaneous dipoles that are able to change polarity rapidly, causing the resulting momentum of the charges to be zero. Now, when the temperature is low enough, the dipole produced is able to generate other dipoles and establish a correlation that causes the atoms to bond at the level of generating molecules.
It is important to understand that the larger the molecule, the greater the intermolecular forces, since the electrons are farther away from the nucleus and are therefore easier to polarize.

MOLECULAR TWINS, HYPOTHETICAL SCENARIOS

To be able to explain the behavior of the different scenarios that occur in our daily lives, have been the result of rigorous observations and experimental processes that have been executed at the scientific level, hence, it is to be expected that scientific assertions or hypotheses occur that over time tend to be refuted and consequently considered as false.
In this sense, in this section of the subject, we will describe one of the hypotheses or theses that over time could be refuted thanks to the scientific advances that have been developed, so that it could be established as a wrong hypothesis.
It refers to the existence of pairs of molecules that differ exclusively in the length of the covalent bonds that make them up, these compounds had been called bond elongation isomers since 1971.
The hypothesis arises as a consequence of a statement by the German chemist Roald Hoffman, who established that it was possible the existence of pairs of compounds with different bond elongations, a statement that the scientific community adopted, in the first instance because prior to Hoffman's statements, scenarios associated with the phenomenon had already been evidenced, also, the influence that Hoffmann had at the time was decisive, since he had been recognized and awarded the Nobel Prize for his contributions to science, so his word had a great weight at the scientific level.

Fig. 4. Representation of dipole moments. Author: Evan Mason
Consequently, the hypothesis ended up being considered as an accepted phenomenon until 1991, where it was determined that the molecules of the compound molybdenum-oxygen could vary their colors from blue to green despite having the same atoms.
At this point, Hoffmann's hypothesis falls down when he was able to demonstrate that the variation in the coloration was due to the presence of impurities or traces of chlorine, this element being responsible for the variations in the color of the compounds studied and consequently, the green color of the compound was nothing more than the result of the combination of the color of the starting compound (blue) and the traces of yellow impurities generated by chlorine, resulting in a green pigmentation in the compound in relation to its impurity-free blue counterpart.
In this way, we realize that scientific theories or hypotheses that allow us to explain a scenario of our daily life, when not proven, may end up being refuted despite the time elapsed.

FINAL CONSIDERATIONS

As we were able to analyze along these lines of writing, intramolecular and intermolecular forces play a fundamental role in the existence of the different states of matter.
Since from these, the molecules adopt different behavior and forms, among which we can highlight the solid, liquid and gaseous states, in turn, the materials resulting from the energetic transformation assumed through changes in pressure and temperature can pass from one state to another, without losing their specific properties.
On the other hand, the knowledge of these forces are the product of research, observation and analysis and are nothing more than models that the scientific world has implemented to explain the existence of phenomena, but in a certain way these hypotheses, if not proven, will be refuted over time, as it happened with the hypothetical cases of the molecular ones.

BIBLIOGRAPHY CONSULTED

[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] McMURRY E., John y Fay C., Robert. (2008). General Chemistry. Fifth edition PEARSON EDUCACIÓN, Mexico, 2009 ISBN: 978-970-26 1286-5.
[3] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). GENERAL CHEMISTRY. Eighth edition. PEARSON EDUCACIÓN. S.A., Madrid.
[4] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCACIÓN, MEXICO, 2011 ISBN: 978-607-32.()793•5. ÁREA: CIENCIAS

OF INTEREST

For more information related to the areas of science, technology, engineering and mathematics, feel free to visit #stemsocial and #stem-espanol, communities that promote scientific advances in these areas.

~~~ embed:1546112935935840257 twitter metadata:eWVyYW5pX2ZlcnJlcnx8aHR0cHM6Ly90d2l0dGVyLmNvbS95ZXJhbmlfZmVycmVyL3N0YXR1cy8xNTQ2MTEyOTM1OTM1ODQwMjU3fA== ~~~
The rewards earned on this comment will go directly to the people( @lindoro ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for this nice post, which I enjoyed to read.
As a side note (let’s start with a side note), the separators do not display on condenser (hive.blog), whereas they are correctly shown on peakd.com. Any idea why? Maybe it is related to unnecessary spacing in the HTML code. See here for a snippet of what I get on condenser:
On the scientific side, I was tented to comment on the fact that there exist more than 3 states of matter. However, I remembered that I already did in your previous blog. Therefore, I will pass for once!
Have a nice end of the week!
Greetings friend @lemouth, as always another great observation, thank you for taking the time to value my writing, regarding the issue of separators, I realize the problem in #Hive.blog I already modify the separators and make the respective update.
Thank you for your valuable comment.
Yes, it indeed works now!
Cheers!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Greetings dear @stemsocial team, thank you for your support and appreciation of the scientific work.
Your content has been voted as a part of Encouragement program. Keep up the good work!
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for new Proposal
Delegate HP and earn more
Thank you @ecency friend for the valuable valuation of our publications, it allows us to continue sharing quality material.
Regards