The term ‘reducing sugar’ reflects the fact that some certain sugars can reduce other chemicals. What this means is that the sugar can donate electrons to other substances. A standard test for this is boiling the sample of a reducing sugar with Benedict’s solution, a blue solution that contains copper sulphate. If a reducing sugar is present in the solution, the Cu(II) ions in copper sulphate are reduced to Cu(I) ions, resulting in an orange-red precipitate. Glucose, galactose, fructose, maltose and lactose are all examples of reducing sugars, but sucrose is not. However, after sucrose is boiled with dilute acid to split it into its monosaccharides, it does give a certain result.

Image by Ulrike Leone from Pixabay
Monosaccharides link by means of glycosidic bonds
When two monosaccharides join together in a condensation reaction, the bond between them, a glycosidic link, centres around a shared oxygen atom. Two α-glucose molecules join together to make one molecule of maltose. Sucrose, the familiar sugar we buy in bags, consists of one molecule of α-glucose and one of fructose. Lactose, the main sugar in milk, is a disaccharide that contains β-glucose and galactose.
POLYSACCHARIDES
Starch: Starch is the most abundant storage chemical in plants and it is the single largest provider of energy for most of the world’s population. Starch has the three properties that are necessary for a storage compound. Starch is: compact, insoluble, and readily accessible when needed.
Starch is a mixture of two compounds, amylose and amylopectin. Amylose is an unbranched polymer in which glucose monomers are joined by α-1,4-glycosidic linkages. These bonds bring the monomers together at a slight angle and, when they are repeated many times, a spiral molecule is produced. In amylose, there are six glucose residues in a turn of the spiral. The glucose chains of amylopectin have α-1,4-glycosidic linkages and α-1,6-glycosidic linkages. This allows branching.
Glycogen
In animals, including humans, glycogen is the main storage carbohydrate. Its structure is similar to amylopectin, but it is even more frequently branched. The advantage of this is more ‘ends’ for enzymes to add or remove glucose. In this way glycogen can be built up and broken down quickly, allowing the animal to store energy efficiently and release it on demand. In humans, glycogen is stored in large amounts in the liver and the muscles. During prolonged exercise, when the immediate supply of glucose is used up, the body restores its supplies by breaking down glycogen. If an average person goes without food, his or her glycogen stores last for about a day, but prolonged exercise such as marathon running can use all of the body’s glycogen in less than two hours. When glycogen runs out, the body turns to using its lipid stores. This is why eating less while taking more exercise is the quickest way to lose weight.
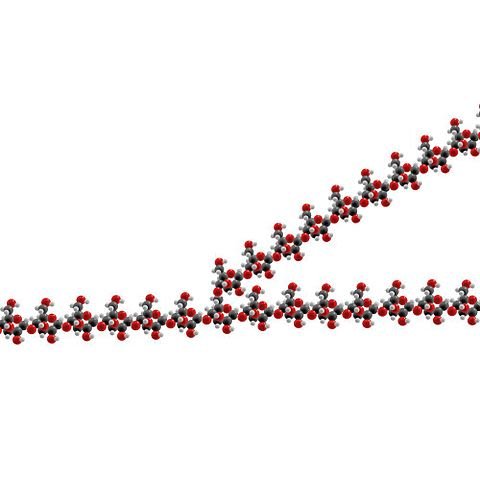
Cellulose
Cellulose is a structural polysaccharide: it gives strength and rigidity to plant cell walls. Individual cellulose molecules are long unbranched chains containing many β-1,4-glycosidic linkages. The molecules are straight and lie side by side, forming hydrogen bonds along their entire length. This results in microfilaments which are boundled into microfibrils and then into strong bundles of chains called macrofibrils.
Cellulose is probably the most abundant structural chemical on Earth, but few animals can digest it because they do not make the necessary enzyme, cellulase. Herbivorous animals, whose diet contains large amounts of cellulose, can deal with it because they have cellulase-producing microorganisms in their digestive system. Humans cannot digest cellulose, but we make good use of it in other ways – it provides the bulk necessary for peristalsis and its strength is used in products such as paper, cotton, Lycra and nail varnish.
Chitin
The exoskeletons of arthropods such as insects and spiders are lightweight, strong and waterproof. These properties are provided by chitin, a polysaccharide that contains many glucosamine units. A glucosamine unit is formed when an amino group (NH2) is added to a glucose molecule.
LIPIDS
Lipids are a varied group of compounds that include the familiar fats and oils. As they are non-polar molecules, most lipids are insoluble in water but soluble in non-polar solvents such as alcohol and ether. Important exceptions are phospholipids, which have polar heads. The emulsion test for lipids, is based on the solubility of lipids in ethanol. Lipids contain the elements carbon, hydrogen, oxygen and sometimes phosphorus and nitrogen. They are intermediate-sized molecules that do not achieve the giant sizes of the polysaccharides, proteins and nucleic acids.
LIPID STRUCTURE AND FUNCTION
The triglycerides, which act mainly as energy stores in animals and plants, are a large important group of lipids. Triglycerides consist of one molecule of glycerol and three fatty acids. The four components join by condensation reactions to form an E-shaped molecule.
Remember that polar and ionic chemicals have areas of positive and negative charge. They dissolve in polar solvents such as water. Non-polar molecules are not charged and do not generally dissolve in water. Lipids are generally non-polar but some can have charged groups, e.g. phospholipids.
The glycerol molecule is common to all triglycerides and so the properties of different triglycerides depend on the nature of the fatty acids. Fatty acids vary in the length of their chain and in the degree of saturation they show. Fatty acids are organic acids. They contain a carboxyl (–COOH) group. In a table showing the chain length of some common fatty acids. Chains of about 14 to 16 carbon atoms are the most usual, but they range from 4 to over 28. A saturated fatty acid has the maximum amount of hydrogen and therefore has no double bonds. Monounsaturated fatty acids possess one C=C bond and polyunsaturates contain more than one.
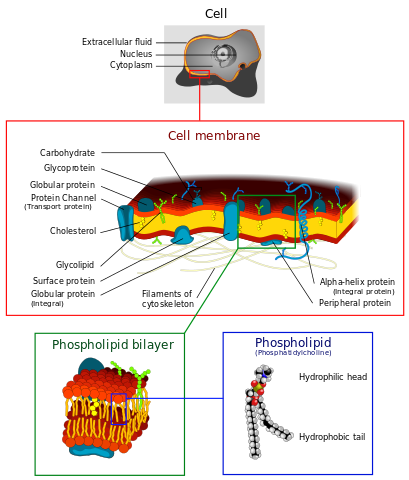
Cell membranes consist of phospholipid bilayers. Dhatfield, CC BY-SA 3.0
Did you know that fat cells have hidden depths?
People used to think that fat cells were just little blobs of fat surrounded by a membrane – which is certainly what they look like under the scanning electron microscope. However, we are now beginning to realize that fat cells are an important tissue in their own right, and that they help to regulate our metabolism. Fat cells produce and secrete a wide range of proteins that act on other cells. Particularly interesting are the hormones such as leptin that act on centres in the brain that tell us to stop eating when we have had enough (but how often do we listen? lol). The importance of leptin was demonstrated in the 1980s when rats that don’t make any leptin were first bred. These animals look normal at birth, but they gain weight rapidly because they don’t know when to stop eating. When they are a few months old, they become grossly fat, resembling spheres of fur with a head, four feet and a tail.
Other hormones produced by fat cells that also help control appetite and feeding behaviour have since been found. The interactions between them are so complex that we don’t yet understand how they fit together. Even so, researchers are confident that progress will be made and that we may have that elusive weight control pill in the next few years or so. The ideal weight for a woman has long been a matter of fashion rather than health.
HIBERNATION
Some animals survive unfavourable seasons when food is scarce, by hibernating. Mammals such as dormice and bats show true hibernation: their metabolic rate is much lower than normal and their core body temperature, breathing and heartbeat rates drop significantly. Core body temperatures of around -5°C have been recorded in hibernating bats. Hibernating animals often have brown adipose tissue, or brown fat as well as white fat. The fat cells in brown fat tissue have a much greater number of mitochondria per cell than white fat and the tissue has more capillaries. The cells have a greater metabolic rate and generate heat, helping to maintain the hibernating animal's body temperature just high enough so that it survives the winter.
Human babies are born with about five per cent brown fat tissue, much of it located at the base of the neck and between the shoulder blades. It helps their bodies maintain normal body temperature. Young babies don't have the muscle tone to shiver until about 12 months, so brown fat is really important in their first year of life. After this, brown fat is lost.
The table below shows some common fatty acids.
| Fatty acid | No. of carbon atoms | No. of double bonds | Abundant in | Melting point/ °C |
| Palmitic acid | 16 | 0 | palm oil | 63.1 |
| Stearic acid | 18 | 0 | cocoa | 69.6 |
| Lauric acid | 12 | 0 | coconut, palm oil | 44.2 |
| Oleic acid | 18 | 1 | olive, rapeseed | 13.4 |
| Linoleic acid | 18 | 2 | sunflower, maize | -5.0 |
Physical and chemical characteristics of different
triglycerides
Triglycerides, which contain longer chain fatty acids and saturated fatty acids, generally form 'hard' fats such as lard and suet that are solid at room temperature. Animal tissues contain a higher proportion of saturated fats than plants. Plant lipids contain shorter chain, unsaturated or polyunsaturated fatty acids and so are 'light' oils that are liquid at room temperature. Unsaturation leads to a lowering of the melting point because double bonds produce kinks in the carbon chain. This increases the distance between molecules by stopping chains from lying parallel and so reducing weak intermolecular forces. This makes the lipid more 'fluid'.
Triglycerides as energy stores
Many animals store energy in the form of triglycerides: gram for gram, they yield more than twice as much energy as proteins or carbohydrates. Triglycerides are highly reduced compounds; they contain many C-H bonds which can yield energy during respiration. If you look at the formula for a lipid, such as C14H26O2 you can see that there is a far greater ratio of hydrogen to oxygen than in a carbohydrate such as sucrose, which has the formula C12H22O11.
Phospholipids
Phospholipids have a similar structure to triglycerides but one of the fatty acids is replaced by polar phosphoric acid. This gives the molecule a polar head and a non-polar tail. When placed in water, phospholipids arrange themselves with their hydrophobic ('water-hating') tails pointing inwards and their hydrophilic ('water-loving') heads pointing outwards. This is vitally important because it results in double layers called bilayers. Phospholipid bilayers form the basis of all biological membranes.
Cholesterol
Many people associate cholesterol with heart disease, but this lipid is a perfectly normal constituent of every cell in our body. As well as eating foods that contains cholesterol, we can also synthesise cholesterol in the liver. The more there is in the diet the less the liver needs to make. However, vegans, who eat no animal products, are easily able to make all the cholesterol they need.
Steroid hormones
Steroid hormones have a similar structure to the cholesterol from which they are made. They include testosterone, progesterone and the oestrogens.
Waxes
Waxes are lipids that are often used to waterproof surfaces, so preventing water loss. The cuticle of a leaf and the protective covering on an insect's body are both waxes. Waxes consist of a very long chain fatty acid joined to an alcohol molecule (not glycerol as in triglycerides). They have no nutritional value because they cannot be digested by lipases (lipid-digesting enzymes).
In my next post, I will discuss more on Steroids, Proteins and Collagen.
Thanks for coming.
REFERENCES
https://www.masterorganicchemistry.com/2017/09/12/reducing-sugars/
https://en.wikipedia.org/wiki/Reducing_sugar
https://en.wikipedia.org/wiki/Glycosidic_bond
https://www.ncbi.nlm.nih.gov/books/NBK22396/
http://www.nutrientsreview.com/carbs/polysaccharides.html
https://biologydictionary.net/polysaccharide/
https://www.ncbi.nlm.nih.gov/books/NBK21190/
https://en.wikipedia.org/wiki/Polysaccharide
https://biologydictionary.net/glycogen/
https://www.ncbi.nlm.nih.gov/books/NBK21190/
https://en.wikipedia.org/wiki/Glycogen
https://en.wikipedia.org/wiki/Cellulose
https://www.britannica.com/science/cellulose
https://www.bbc.co.uk/bitesize/topics/znyycdm/articles/z2d2gdm
https://en.wikipedia.org/wiki/Chitin
https://www.britannica.com/science/lipid
https://en.wikipedia.org/wiki/Lipid
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.