Introduction
 [License: Public Domain]:
Pixabay
[License: Public Domain]:
Pixabay Proteins are the guys responsible for majority of functions in the cell. I know yesterday I said enzymes but are enzymes not proteins ? The central dogma always tells us the same thing and yes, I make it a habit to bring it up every single time. Well, you cannot blame me as this pathway is very essential in biochemistry as well as molecular biology and it embodies life from the genotypic to the phenotypic level. You remember some time back I talked about Mr HIV defiling the central dogma by going from RNA back to DNA. Well, today we move a step forward. I mean we go beyond the central dogma, which tells you how DNA becomes RNA and RNA becomes protein, and focus on what happens after the amino acid sequence which is technically the protein in its primary state is formed. I explained the different levels of protein organization here.
What is Protein Folding Really All About ?
 A Schematic Representation of The Four Levels of Protein Organization [License: CC-BY-SA 4.0, Author: Scurran15]:
Wikicommons
A Schematic Representation of The Four Levels of Protein Organization [License: CC-BY-SA 4.0, Author: Scurran15]:
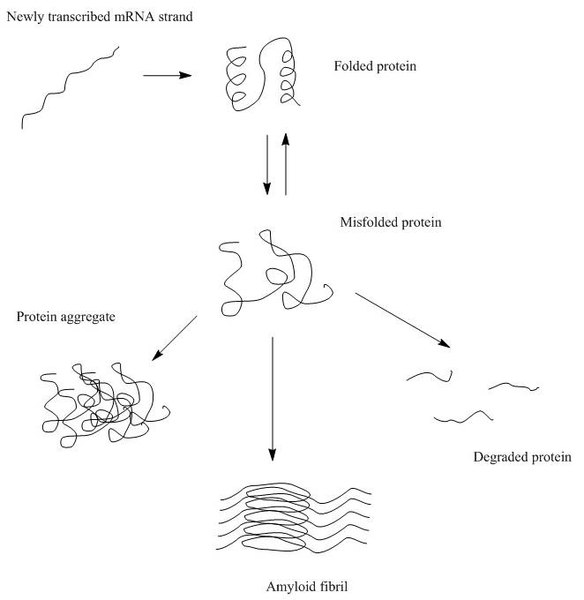
Wikicommons So after the amino acid sequence, which is the primary structure of the protein, is formed, the protein resorts to folding to achieve the lowest energy level possible as well as assume a compact definite structure. The protein assumes a three-dimensional structure and this structure is quite essential for its function. It is in this conformation that a protein can interact best with other molecules, carrying out its function ultimately. An enzyme would not bind a substrate if it is not in its 3-D folded form because its active site would not even be formed. So this conformation is very vital for a protein’s function. So apparently, this means that proteins must fold correctly in order to function. The cell is quite very efficient that out of a million proteins folded; only 1 could be misfolded and even with that, cells have a way to correct that. Most proteins have amino acids so randomly arranged that they cannot assume their secondary/tertiary structures on their own. This is where special molecules (which are also proteins) called chaperones come in. These molecules help proteins fold and make sure unfolded proteins are made to fold. Chaperones are expressed more during heat shock response seen in the nucleus and cytosol and unfolded protein response seen in the endoplasmic recticulum. These are ways these compartments try to reduce the effects that accompany protein misfolding.
So Why Do Proteins Misfold ?
 Protein Aggregation [License: Public Domain]:
Wikicommons
Protein Aggregation [License: Public Domain]:
Wikicommons Perfection is only but a wish so every system is prone to errors. Cells mistakenly incorporate wrong nucleotides into a growing DNA molecule so incorporating wrong amino acids into a growing protein molecule should not come as a surprise. This often leads to protein misfolding because when A interacts with A normally for a given protein molecule but somehow it is presented with B, it tends to just work with what is has and that gives rise to non-functional structures. At times, amino acids in a protein molecule bind wrongly, maybe head to head instead of head to tail (please this is only an analogy, it is never this way in real life situations), giving rise to a wrongly folded structure. Protein folding occurs in several compartments viz-a-viz the mitochondria, peroxisome, cytosol and the nucleus. These compartments have ways of handling protein misfolding but things still go south anyway.
So How Do Diseases Occur When Protein Misfold ?
 Prion Replication [License: CC-BY-SA 3.0, Author: Joannamasel at English Wikipedia]:
Wikicommons
Prion Replication [License: CC-BY-SA 3.0, Author: Joannamasel at English Wikipedia]:
Wikicommons When proteins misfold, they form aggregates. Most times, proteins change their native conformation and this is hugely implicated in their formation of aggregates. Most proteins assume the alpha-helix form as their native conformation but at times, as a consequence of misfolding they assume the beta-pleated sheet form. The beta-sheets are more likely to form aggregates since they possess inter-chain bonding (bonds are formed by two beta-pleated sheet molecules) as opposed to the alpha-helices which possess intra-chain bonding (bonds are formed within the same molecule). This is probably why most proteins exist as alpha helices. There are proteins that exist as beta-pleated sheets anyway. An example is the immunoglobulin. The aggregates formed by misfolded proteins can be referred to as amyloids or amyloid deposits. They are known to induce oxidative stress, block cellular functions and turn other normal proteins to toxic proteins through a process known as seeding. The deposition of amyloids and degeneration of tissues and organs as well as the development and progression of diseases is all just a correlation and does not exactly indicate causation but it implies however that these amyloid deposits may very much be responsible for the diseases.
The Common Diseases That Result From Protein Misfolding
 Neurodegradation [License: Public Domain]:
Pixabay
Neurodegradation [License: Public Domain]:
Pixabay The very first and commonest consequence of protein misfolding is sickle cell anaemia. Here, there is a substitution of glutamate for valine in the B chain of haemoglobin causing a change in conformation and subsequently, presenting as a disease. These haemoglobins appear sickled and are incapable of transporting oxygen effectively. They also damage blood vessels due to they fect that are not as flexible as normal haemoglobin. It is known that there is currently no cure for this but with CRISPR/Ras9 technology, anything could be possible. The mutated genes could be corrected and we could end up having SS individuals becoming AA individuals but this is just science imagining possibilities.
Another example of protein misfolding causing disease is seen in Alzheimer’s disease. Here, aggregates of a misfolded protein called amyloid-B is seen in increased concentrations. Tau proteins are also seen in elevated concentrations. It is important to note however that the mechanism underlying alzheimer’s disease is not fully understood so elevated concentrations of these proteins does not directly indicate that they cause alzheimer’s but rather show a correlation only. In this disease, there is degeneration of nerve cells and this neurodegeneration begins way before amyloid-B can be detected in brain tissues and even before symptoms of memory loss appear. This makes understanding this disease even more difficult than usual.
Another incidence of protein misfolding resulting in disease is seen in Parkinson’s disease (PD). Here, a protein called α-Synuclein is seen forming aggregates in nerve cells and so is implicated in the death of neurons noticed in PD. This could also be responsible for the tremors and rigid movements noticed in PD. Yet again, this is all just a correlation and does not exactly indicate causation.
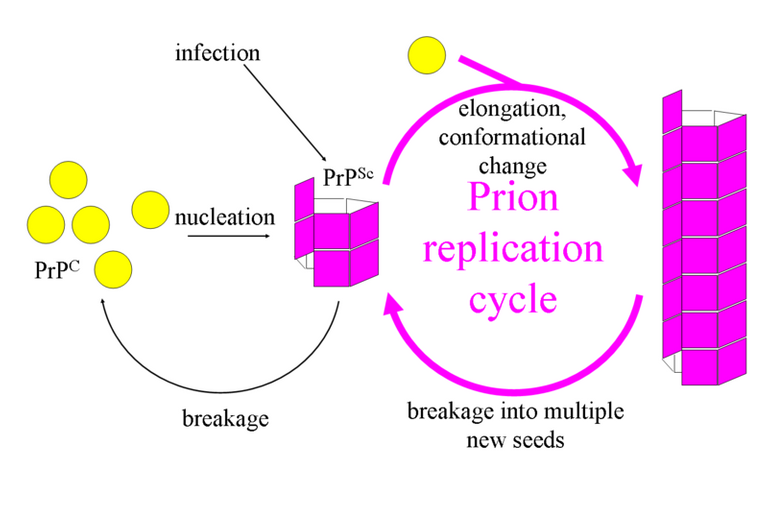
The Transmissible Spongiform Encephalopathies (TSEs) are also as a result of protein misfolding. They are mostly caused by a protein called prion. This protein invaginates the brain and begin to change a similar protein (PRPc) found in the brain to its conformation. They begin to form aggregates and brain tissue degeneration occurs. Scrapie found in sheep and Creutzfeldt–Jakob disease found in humans are examples of TSEs.
Protein Misfolding Diseases Have No Cure But Is There a Way Out ?
 [License: Public Domain]:
Pixabay
[License: Public Domain]:
Pixabay Therapeutic drugs which target the steps of protein misfolding could be designed. This would ensure that chances of proteins assuming the wrong conformations are low. Drugs that induce the production of positive chaperones could also be designed. This will go a long way in ensuring that proteins fold properly. Normally, cells try to degrade misfolded proteins as a quality control mechanism but in some cases, this mechanism is inhibited or altered as a consequence of prion diseases. Drugs that enhance the activity of proteases which degrade these misfolded proteins could be designed. Protein clearance is also inhibited in these conditions so drugs that could promote protein clearance could be designed. Studies as well as preclinical trials have been carried out aiming to cure protein misfolding diseases but most of them fall short of clinical trials while some are still about to undergo preclinical trials. Hopefully, we begin to find suitable cures and maybe a vaccine.
References
https://www.nature.com/scitable/topicpage/protein-misfolding-and-degenerative-diseases-14434929
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3882043/
https://en.wikipedia.org/wiki/Proteopathy
https://www.sciencedirect.com/science/article/pii/S0014579301024863
https://febs.onlinelibrary.wiley.com/doi/full/10.1111/j.1742-4658.2006.05181.x
https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-017-0077-5
https://www.future-science.com/doi/pdf/10.4155/fso.15.38.
Image Sources
All images are licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
Protein misfolding -- I once heard of this at a sickle cell programme organised by an NGO late last year, never knew it was responsible for couple of other diseases which have no cure.
Great stuff in here @kingabesh -- reading on prions
So, in essence, misfolding of proteins could be hereditary. interesting.
Yep and also sporadic.
Hi @kingabesh!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @kingabesh! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Do not miss the last post from @steemitboard!
Participate in the SteemitBoard World Cup Contest!
Collect World Cup badges and win free SBD
Support the Gold Sponsors of the contest: @good-karma and @lukestokes
Interesting post! There is actually a new therapy in development that can specifically target proteins for activation and then destruction in the proteasome. These things are called PROTACs and I think some Princeton scientists came up with it, might be interesting for you!
As for prions, they really do mess up the germs theory of disease because they can cause other proteins to misfold. I cannot remember exactly how, but they do evade the proteasome system and do not cause any symptoms for years. They build up before reaching a critical amount, when the disease breaks out. It is pretty funny though, that while cause a disease (Prp Sc) the Prp wildtype seems to be serving a purpose. Anyways, cool post. Cheers!
Interesting contribution man. I’m actually glad to hear about the PROTACs and yes, prions mess up the germ theory of diseases. Glad to have you stop by mate.
Seems this is serious than I thought. On seeing the heading, I felt you're about to talk about "jedi-jedi" that taking too much protein causes.. But then, protein misfolding seems more completed and more alarming for it being hereditary.
Nice work here..
Thanks man. Proteins can mess things up man. Glad to have you stop by again.
It's a pleasure always fam!
Thanks man. Proteins can
Mess things up man. Glad to have
You stop by again.
- kingabesh
I'm a bot. I detect haiku.
Fuck you, silly bot. Here, a flag.
😂😂😂😂😂No mercy