They shape you and you shape them. The talk between your immune cells and your bacteria can decide your shape.

Adapted from
Dancing mouse | CC0 1.0
Fat mouse by Clker-Free-Vector-Images | Pixabay
Source | Created by Nogas1974
[CC BY-SA 4.0](CC BY-SA 4.0)
I have touched upon the topic of the gut microbiome (the bacteria that lives in your gut) earlier. I have talked about how they affect your metabolism. I have also discussed how the microbiome diversity is reduced in obesity and how it can contribute to type 2 diabetes. We have seen how bacterial community that lives in us can be shaped by our genetics, our diet and by the phycological stress we face. There are reports on how microbiome trains our immune system, affect our gene expression, and may even control our food cravings. However, what remains elusive is which microbial communities among the 1000s that live within us makes us obese vs which makes us thin. More importantly, what possible mechanisms could change the kind of communities that resides within us. In the paper I discuss today, the authors have discovered one such mechanism. They talk about how if the one kind of bacteria vs other dominates in our gut, it can cause obesity. They also discovered the role of T cells in regulating the type of bacteria that lives in the mouse gut.
The paper
The paper we will discuss today is titled T cell–mediated regulation of the microbiota protects against obesity published by Petersen et al., from June Round's and W. Zac Stephens' labs at Utah. It is published july 2019 edition of Science.
So how does the story start

Adapted from Mouse cheese by OpenClipart-Vectors and Taco mouse by AnnaliseArt
Pixabay
Once upon a time, there were researchers working with germ-free mice. These are the mice born and brought up in a completely sterile environment and have never seen a bacteria in their life. Funny enough that even when these mice would eat a lot more food than their germ containing counterparts they would not gain any weight (Bäckhed et al., 2004). No, nothing at all. However, when they were given any germs to colonize their gut they would. If you gave them germs from obese humans they would even become obese. But when give them germs from lean humans, they won't gain as much weight (Turnbaugh et al.,). Moreover, one can take normal mice, treat them with antibiotics and give them either microbiome from obese or lean mice and alter how they gain weight (Ellekilde et al., 2014). In fact, it seems that in mice certain kind of bacteria is required in the gut for effective weight loss on a calorie-restricted diet (Wang et al., 2018). Now, all this is very interesting, it means there are some bacteria in the gut of obese humans that made them obese.
In fact, from sequencing microbes from obese individuals we know that there is reduction in the richness of their microbiome (Chatrlier et al., 2013). The species composition of obese and lean people show characteristic variations. Even if you were not looking at all the species in there, the overall functional profile of microbiome is different in obese vs lean individuals. For instance, gut bacteria in obese individuals are more potent in producing butyrate and other short-chain fatty acids, which are implicated in obesity (Chakraborti, 2015). Then, Zierer et al., 2018 gives a detail description of how age, sex and obesity can be correlated with differences in metabolites produced in the gut.
Anyhow, obese people have variant microbiome and all is fine. But the question arises that what changed this microbiome. Of course, there could be a genetic link to kind of microbiome you carry. It could also be your diet, your lifestyle, phychological stress, or just ageing that took the composition to the wrong turn (see my previous blog). Other than this the state of the immune system itself can alter the microbiome. From the day we were born our immune cells and gut microbes have been talking to each other. Now, imagine a situation where at some point in your life these talks get messed up. What if immune system because of age, stress or diet fails to keep a check on friends vs foes?
While on one hand obesity is associated with chronic low-grade systemic inflammation (see previous blog), on the other hand, obesity also makes us prone to some infections. In this regard, Tanaka et al., 1993 showed that T cell and B cell responses are compromised in obese individuals. Your B cells produce different classes of antibodies - IgG, IgM, IgA and IgE. Obesity is also associated with a reduction in secreted IgA levels in the gut mucosa. Though IgA deficiency in the gut doesn't have dire consequences it seems to have an effect on the composition and functional profile of microbiome (Pallaro et al., 2002, Fadlallah et al., 2018). It has been demonstrated in mice studies that IgA has a crucial role in the gut in maintaining healthy metabolism (Luck et al., 2019). Recently, it has also been shown that T cells carry toll-like receptors (these are the receptors that senses microbial patterns), which act through an adaptor protein called Myd88. This signalling is required for naive T cells to differentiate into T follicular helper cells (Tfh cells). Tfh cells signals B cells in lymph nodes of gut to start producing IgA. Perturbing this pathway by knocking out Myd88 in T cells causes dysbiosis in mice gut (Kubinak et al., 2014).
Nevertheless, what is not known is what is the mechanism by which the deficiency in Tfh cells affects IgA, change the microbiome and cause health consequences. This is what the current paper focuses on.
The story
Mice lacking a gene for Myd88 in T cells gains weight with age.
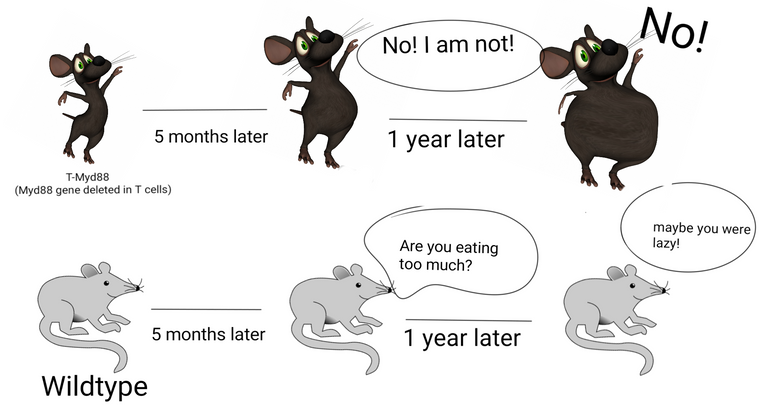
Illustrated by @scienceblocks using Image by da5id1 | public domain and Dancing mouse | [CC0 1.0].
The current story goes something like this. The researchers had a mouse which had Myd88 gene deleted specifically in T cells. They call it T-Myd88 mice. And the mice that have this gene intact, we are going to refer them as wildtype mice. The T-Myd88 mice seem to do fine for most of its life. But, hell breaks lose by the time it turns 5 months old. It starts gaining more fat. By the time its 1-year-old its at least twice as heavy as its wildtype cousins. It has at least 50% body fat percentage and a huge increase in visceral body fat. Oh, and it also develops obesity-associated fatty liver, chronic low-grade inflammation in adipose tissue and develops insulin resistance. Pretty much like obesity-associated pathology in humans.
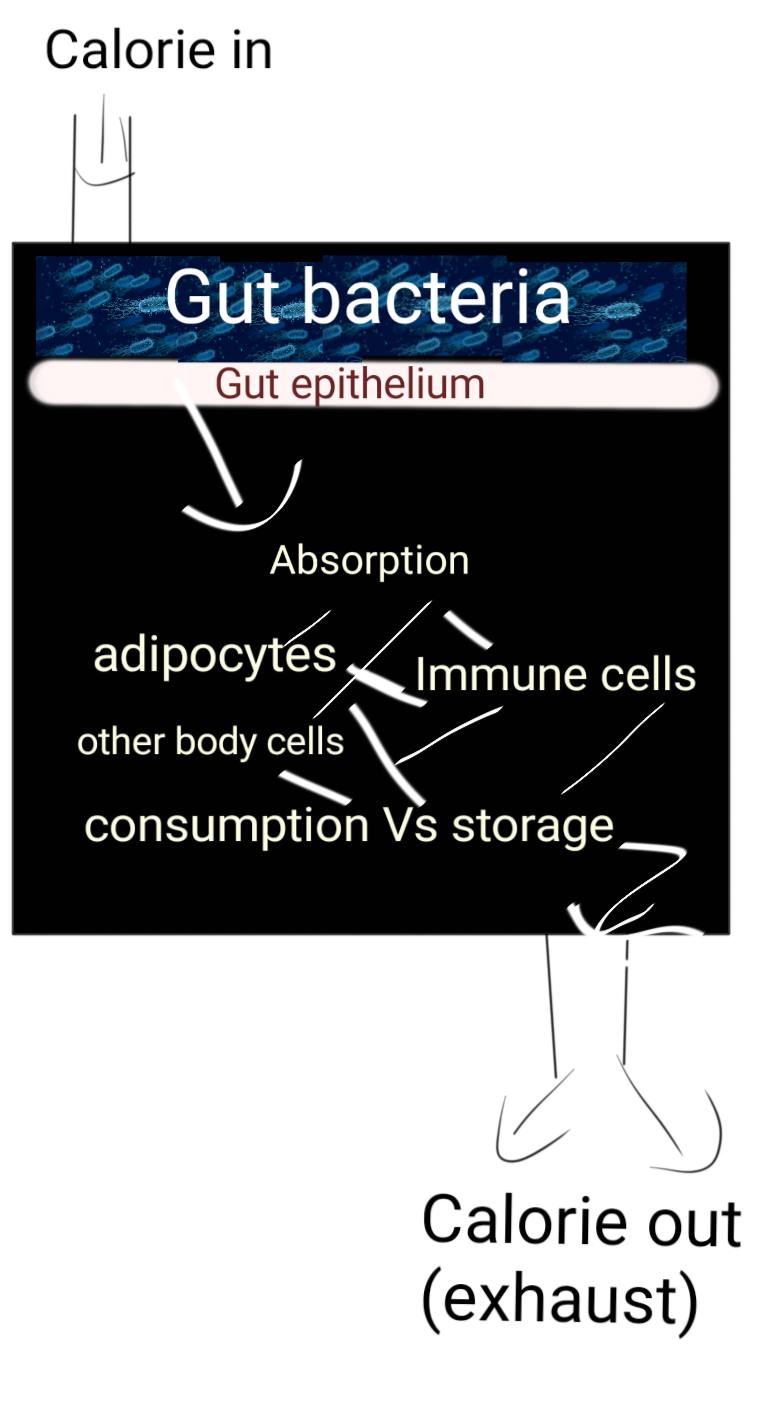
Illustrated by @scienceblocks.
Bacteria by qimono | Pixabay
T-Myd88's wildtype cousins perhaps tease it for eating too much or being too lazy. But guess what's funny. It ate the same chow as wildtype mice. In fact, at 2 months of age, it consumed a lot less food. By one year of age, it consumed no more calories than the wildtype guys. And to check whether these mice are being lazy, the researchers kept them in these special cages which can measure the metabolic activity of the mice by measuring its oxygen consumption. They also checked of these mice are moving around any less. But nada! They moved around as much as the wildtype guys. Though young mice showed slightly less energy expenditure, this difference did not persist as they grew older. So, neither the energy entering through the mouths of T-Myd88 nor the calories being burnt by it could explain the weight gain in these mice.
However, a lot happens between food entering the mouth and being burnt in the body. It matters how it is processed by the microbiome, how it is absorbed and how much of it is sent for storage vs consumption. Since T cells were knocked out a Myd88 gene, there could have been two explanations. Either the mutated T cell, by themselves, were changing the absorption of food or its storage, or they were indirectly doing so by interacting with the gut bacteria. It's a simple thing to test if its bacteria that is causing the weight gain then killing them by antibiotics in T-Myd88 mice should inhibit weight gain. And so it does.
Ok so now that they knew that it is most likely the bacteria that is making the T-Myd88 mice fat, the next question is how? Which bacteria is lost or gained? What functions are lost or gained? What are they doing? And what caused the loss or gain or these bacteria?
The war for colonization - Clostridia Vs Desulfovibrio
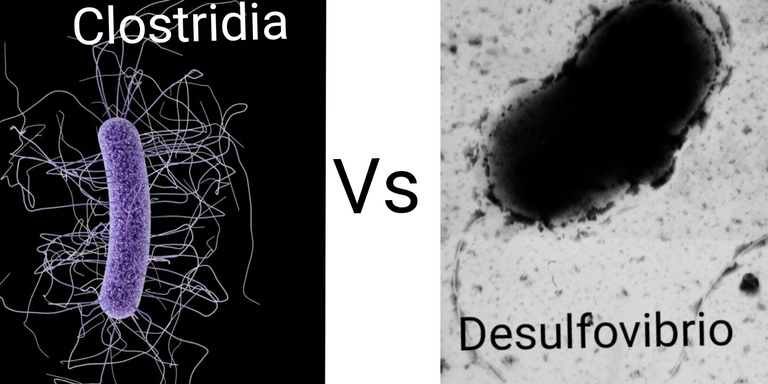
Clostridia by CDC/ James Archer | CC BY-SA 4.0. Desulfovibrio vulgaris by Graham Bradley | Public domain.
To know which bacterial species are lost or gained the authors sequenced the microbiome of T-Myd88 and wildtype mice. The authors did both 16S rRNA sequencing (that's ribosomal RNA to know the species details) and transcriptomics (to know the functional aspect by looking at gene expression). They found that there was an overall reduction in species richness in T-Myd88 mice. They also pinpointed that T-Myd88 have a reduced diversity of species and functions, esp relating to the class of bacteria called Clostridia
Communicable Obesity?
Illustrated by @scienceblocks using Image by da5id1 | public domain and Dancing mouse | [CC0 1.0]. Poop by openclipart-vectors | Pixabay
Well, since mice literally eat shit, they hypothesized that if they keep wildtype and T-Myd88 mice in the same cage then they should be able to transfer their microbiome to each other's gut. This would either make T-Myd88 slim or wildtype fat. Well guess what, WT that stayed with T-Myd88 mice gained weight and their microbiome profile became similar to that of T-Myd88 mice. Which meant they too lost many species of class Clostridia.
And the winner is - Desulfovibrio
But here is the catch the microbiome of these two mice took 3 months to look similar, but the weight gain wildtype mice started as early as 3 weeks after starting a live-in with T-Myd88. So what was up with that? Seems like there was another bacterial family called Desulfovibrio that was first to take over the gut of partner wildtype mouse. So the authors thought that perhaps this is the guy responsible for making wildtype gain weight and also lose Clostridia over time. Desulfovibrio is the bacteria that degrade mucin (glycoproteins in mucous membranes) and produce hydrogen sulphide as a result. These sulphate reducing bacteria, which produce H2S and have also been found in the gut of humans with type 2 diabetes (Qin et al., 2012).
Hence, to test whether Desulfovibrio were sufficient to cause a reduction in Clostridia, they gave Desulfovibrio desulfuricans to pathogen-free wildtype mice. And there it was, 1 week later these mice lost some families and genus of class Clostridia. Then to further confirm of the loss of Clostridia is a direct effect of Desulfovibrio, authors colonized guts of germ-free (GF) mice (remember that GF mice have nothing in their gut, to begin with) with Clostridia and then gave them Desulfovibrio. And they were right, Desulfovibrio hates Clostridia. However, if they were right then giving fresh Clostridia to T-Myd88 mice should stop them from gaining weight. And, it does.
So why does Clostridia helps in keeping the mice lean and why does Desulfovibrio make them fat? Well maybe because some bacterial products leak into the gut of T-Myd88 mice that does not leak in wildtype? What could those products be? Maybe some inflammation causing products? But authors did not find a difference in this regard between wildtype and T-Myd88 mice. Moreover, anti-inflammatory drugs did not rescue weight gain in these animals. However, what authors did find is that T-Myd88 mice did show a difference in gene expression for genes involved in lipid metabolism and lipid absorption. Of these genes, the most interesting was the CD36 gene in the gut epithelium. This gene in gut epithelium downregulated by Clostridia, while Desulfovibrio upregulated its expression. The increased expression of CD36 caused long-chain fatty acids to pass through gut epithelium with ease. Hence, the weight gain in T-Myd88 mice can be blamed on increased absorption of long-chain fatty acids from the gut.
However, at the moment it is not clear which property of long-chain fatty acids is causing obesity. Is it just caloric value of these fatty acids or they also influence fat storage and metabolism in some way. I guess time would tell.
It all started with the T-cells
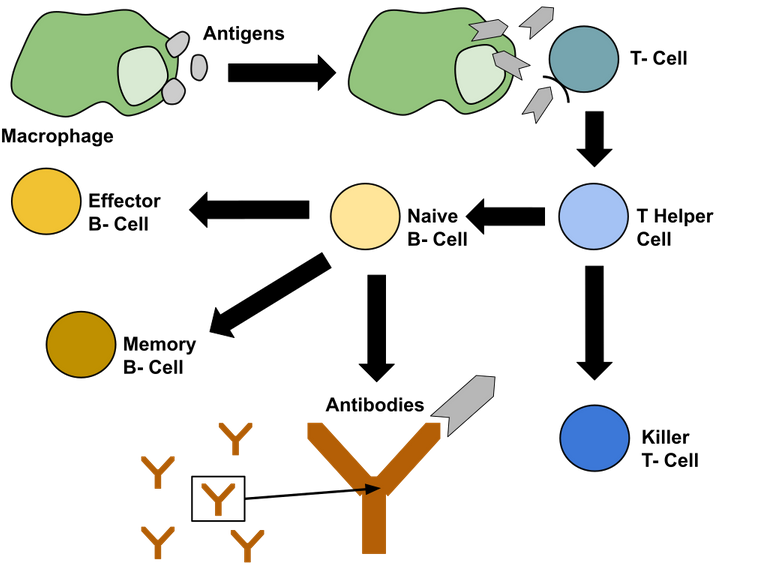
Image by Sstern19 | CC BY-SA 4.0
So far so good. But what is the evidence that T cells have any role to play in it? I mean sure, that the obesogenic microbiome arose in T-Myd88 mice. But does it mean that T-cells are required for maintaining healthy microbiome? Does the lack of properly functioning T cells favourable to the persistence of microbiome associated with obesity?
Well, normal T follicular helper cell and B cell interaction are important for proper IgA production that targets the correct bacteria. The hint for this comes from the experiment in which authors inject T helper cells from either wildtype mice or Bcl6 knockout mice into TMyd88 mice. Bcl6 knockout T cells can't differentiate into Tfh cells, and therefore can't educate the B cells to produce IgA. But T helper cells from wildtype mice can. The authors show that injecting wildtype T cells rescues weight gain in TMyd88 but injecting T cells from Bcl6 knockout background does not.
To test how microbiome is affected by T-cells authors took the Tcrb knockout mice (Let's call it TcrbKO). The mice lacking this gene, do not make T cells whatsoever. They killed the original microbiome of TcrbKO mice with antibiotics and then gave it 1:1 mixture of wildtype and T-Myd88 microbiome. They then injected them with T-cells, either from wildtype mice or from T-Myd88 mice. The mice that got T-cells from T-Myd88 mice gained much more weight. They also saw that only 10% of the microbiome received by TcrbKO mice was coated with IgA. But when they were given wildtype T cells >40% of microbiome got coated with IgA antibodies. However, IgA coating only increased to some 30% in mice receiving T cells from T-Myd88. Over the course of time, TcrbKO mice that got T cells from TMyd88 mice Lost Clostridia, while Desulfovibrio took over.
However, what is more interesting is that despite IgA coating slightly less bacteria overall, in mice that got T-Myd88 T cells, there were some bacteria that got targeted more, while others got targeted less. While there wasn't much difference targeting of Desulfovibrio in animals injected with T cells from either wildtype or T-Myd88 mice. So what does it say? It probably means that IgA antibody has more to it than targeting a bacteria for destruction. The authors argue that it can either favour or disfavour the growth of certain microorganisms. It can change the genes they express and metabolites they produce. However, at the moment it is not clear that why MyD88 lacking T cells causes B cells to make IgA that targets different species. It would certainly be an interesting topic to be explored by future studies.
Summary and take home message.
To summarize it, it seems that in their model it was wrong of the lean mice to hold the mutant mice responsible for gaining more weight. It's not like mutant mice were eating more or burning less. Rather, the mutant mice lacked a protein called MyD88 in its T cells. This prevented the T cell to talk properly to B cells. Because of this miscommunication, the B cells did not produce the right kind of IgA antibody. Since IgA did not go and bind to right kind of bacteria in the gut of this mutant mice the good guys of the bacterial community, Clostridia were lost. Instead, the gut of mutant mice was overtaken by another bacteria called Desulfovibrio. In fact, when the lean non-mutant mice started living with mutant mice, they became fat as well. Well, because they ate shit of mutant mice which had Desulfovibrio in it. Colonization by Desulfovibrio seems to antagonize the survival of Clostridia.
So if input and output of calories were similar in both mutant and non-mutant mice, how was a change in microbiome making them fat? It turns out Clostridia and Desulfovibrio have a different effect on gene expression of the host. They have opposite effects on the expression of genes that regulate lipid absorption and metabolism. In the presence of Desulfovibrio, the gut absorbs more long-chain fatty acids than normal.
However, what I don't like about the paper is that it left too many loose ends open. It gives rise to more questions than answers. For instance, why would IgA end up targeting different bacteria based on mutant T cells? What is exactly long-chain fatty acid doing in there? Is it just the calories of these fatty acids or it affects the function of muscles and adipose tissue as well. How does it affect insulin resistance and if the inflammation of adipose tissue is a direct consequence of these fatty acids? I know future studies might address these questions but I am greedy to know more. Aren't you?
Moreover, the germ-free mice when given obesogenic bacteria orally did not gain weight. The authors argue that this is because germ-free mice had an intact immune system. But so did wildtype mice that were put in the same cage as mutant mice. Why eating shit from mutant for 3 weeks caused weight gain in the normal mice? It's possible that there is more to eating shit naturally than getting bacteria artificially. I wish authors dwelled a little more on this part. I wish they answered that why despite having functional Tfh cells, Desulfovibrio overtook Clostridia in wild type mice? Maybe an experiment where they gave the purified bad bacteria for prolonged period to separated wild type to see if it is still able to colonize the gut and make wild type fat.
Anyway, I will stop talking here and instead would love to hear your comments. What do you think about this paper? What questions came to your mind? Do you think there is another experiment that authors should have done?
Also, do let me know if you understood the paper with ease or if you struggled with the jargons. Did you enjoy it or would you rather prefer if it was simpler than this?
A bit about journal club series
This is a new series I am starting. In this series rather than discussing an entire field say about diabetes, CRISPR or ageing, I will summarize that paper I liked a lot, and we can have a discussion on it. We often do this in the lab, so I thought why not on steemstem? In comments do let me know if you liked the idea and approach, and if you think I should add or change something. It would be nice of you to put up questions on the paper and fuel discussion. Also, if you like it, I would encourage you to write about your favourite papers.
About steemstem
But, before I go I would like to mention about the steemstem platform. Well, if you love reading and writing interesting science articles @steemstem is a community on steem that support authors and content creators in the STEM field. If you wish to support steemstem do see the links below.
You can vote for steemstem witness here -Quick link for voting for the SteemSTEM Witness(@stem.witness)
Quick delegation links for @steemstem
50SP | 100SP | 500SP | 1000SP | 5000SP | 10000SP
Delegating to @steemstem gives an ROI of 65% of the curation rewards.
Also, if you have any questions regarding steemstem, do join the steemstem discord server.
You can DM me on discord, I have the same handle - @scienceblocks. Also if you are not a steem user, and reading this blog inspired you to start your science blog, find me on discord and let me know about you. I can try and help you navigate your way through steem.
References
Wang et al., 2018. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice.
Chatelier et al., 2013. Richness of human gut microbiome correlates with metabolic markers.
Type 2 Diabetes - The gut feeling that you might be a bacteria
Fadlallah et al., 2018. Microbial ecology perturbation in human IgA deficiency
Luck et al., 2019. Gut-associated IgA+ immune cells regulate obesity-related insulin resistance
Amazing @scienceblocks!
This has been a topic that has sparked my interest for quite a while now. And I appreciate you pointing out here that psychological stressors can also be counted as factors that disturb the communities that reside within our bodies.
This is indeed a great, engaging way to lay out one of the things that happens in between the moment food is ingested, and burnt in the body. I have also been curious about the many processes involved in the alteration of the microbiome! And the understanding of T cells role in regulating the different types of gut bacteria is new to me :)
Those poor, little T-Myd88 mice. Riddled with inflammation, and on top of that teased by their cousins! No wonder why they became vindictive and pushed their counterpart to eat poo! :D
Your articles are becoming better and better @sicenceblocks. You manage to make them very enjoyable to read! Plus, the artwork is so cute and funny!
Oh, and before I go:
.I want to live in a completely sterile environment :P
Psychological stressors are definitely showing up.to play a crucial role in our metabolism and even dictating our shape. Esp the childhood stressors. This topic certainly needs our attention, given how easily we disregard stress in life. I know these relatives who have autoimmune diseases running in the family, along with all the anxiety I see in their behaviour. They would blame things on everything except recognising the anxiety issue over trivial things. It's only recently that I showed them evidence of how stress can accelerate these diseases. And began to actively work on being calm and they now think that the disease flare ups are not as much. I know this isn't the evidence enough for us, but they are happy and doing well. So be it.
Lol, not that counterpart minded eating poo, but it know the vendetta of T-Myd88.
T-Myd88 as V
And, thank you for all the motivating comments that you make. If not for feedback I get from you guys, I would clueless how to improve and make articles enjoyable. 🙂🙃
Posted using Partiko Android
Ahahahahahah ahahahahaha !! Love it. Way to begin my morning !! 😂
Ps: Pleased to hear your relatives are doing well now :)
I wish you and your wife a wonderful day ! ☀️☕️
There was no jargon. A fascinating study...something along the lines of "you are what you eat" :))
Your drawings are ideal. They not only break up the text and illustrate different points, but they are delightful.
My favorite part (because I am a skeptic) was when you raised questions. Too neat for you, as you say. Loose ends not tied up:
As I read my mind strayed to poop pill therapy used to treat Clostridium difficile. It's a completely different target, but still the transfer of gut bacteria has interesting consequences.
Great article. You do amaze :)
This article is much clearer than the previous one. Really!
In other words, to check whether I have well understood: if we are missing some good bacteria in the gut, this means there is room for no so good bacteria to take over. And the presence of the good guys were controlled by this missing gene. Am I right? :D
Yes! Thanks @lemouth. That's indeed the message. One more thing though, the bad guys in large numbers were able to take over the good guys even when gene was intact. There was something about living together with mutants!
Lol! Mutations are transmissible. Yes indeed, I forgot to mention this part (with a very nice cartoon ;) ).
That was a thorough post, thank you!
This post has been further promoted on Facebook, Instagram, Reddit and Twitter by the SteemSTEM team!
I just saw your Facebook post. I am going to check other platforms. Thanks for all the support and encouragement. The steemstem community is doing a great job.
Posted using Partiko Android
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Hi @scienceblocks!
Your post was upvoted by @steem-ua, new Steem dApp, using UserAuthority for algorithmic post curation!
Your UA account score is currently 3.657 which ranks you at #5876 across all Steem accounts.
Your rank has not changed in the last three days.
In our last Algorithmic Curation Round, consisting of 133 contributions, your post is ranked at #32.
Evaluation of your UA score:
Feel free to join our @steem-ua Discord server