It has long been noticed that the speed of chemical reactions can be modified. sometimes very intensely, by the intervention of bodies whose chemical composition remains unchanged during the reaction. Catalysis is the term for this occurrence. In general, it is about the acceleration of the chemical transformations (positive catalysis), although one knows cases of slowing down of the reactions (negative catalysis). By introducing platinum foam into a mixture of oxygen and hydrogen, the reaction is accelerated to the point of causing an explosion. Adding a few drops of water to a mixture of iodine and aluminum powder causes intense combustion, ie a significant acceleration of the formation of aluminum iodide. These are examples of positive catalysis. On the other hand, the oxidation of certain unsaturated compounds used in the composition of rubber can be slowed down by adding certain amines, so this is an example of negative catalysis.

Source: Pixabay
In what follows, we will mainly study positive catalysis, which plays an extremely important role in the chemical industry and whose mechanism can be more easily defined.
- How does the rate of chemical reaction change when a catalyst is used?
The rate of a reaction can be changed by varying the activation energy or the spatial position of the reacting molecules in a direction that is favorable to the progress of the reaction. Catalysts act on both of these factors. Most often the catalyst reduces the activation energy, but cases are also known where the acceleration of the reaction is due to the catalyst modifying the spatial position of the molecules.
The decrease in the activation energy produced by the catalyst in a reaction comes from the fact that the latter provides it with a new path whose activation barriers are lower.
The fact that the reaction follows another path means that the catalyst forms with the reactants intermediate compounds whose decomposition regenerates the catalyst which enters the reaction again. It is important to realize that the catalyst does not move the equilibrium position, only favoring the achievement of equilibrium, and exerts an equal influence on the rate constant of both the direct reaction and of the reverse reaction.
- How does the rate of chemical reaction change when a catalyst is used?
- This action is precisely made possible by the participation of the catalyst in the formation of the intermediate compounds; the nature of the latter is therefore interesting to know. One can even say that the study of intermediate compounds is one of the main problems of the modern science of catalysis. In some cases, unfortunately few in number, the composition and properties of the intermediate compounds can be determined. Calcium carbonate acts as a catalyst for the decomposition reaction of acetic acid into acetone, water and carbon dioxide. In the catalytic reaction the intermediate product is calcium acetate. This can be proved by heating pure calcium acetate; by raising the temperature, acetone and calcium carbonate are obtained.
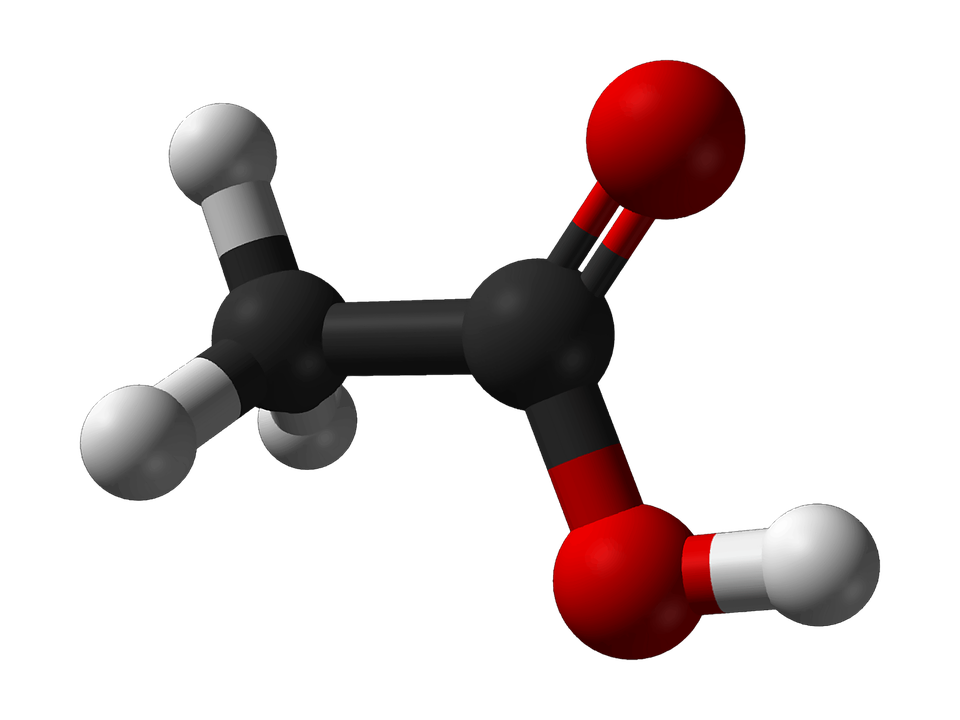
Acetic acid molecule. Source: Pixabay
The most direct augmentation, making it possible to decide on the correctness of such and such a diagram of a catalytic reaction, is that which consists in isolating the presumed intermediate compound and studying the kinetics of its decomposition. If, by proceeding in this manner, the researcher obtains the same end products and at the same rate as in the actual process using the identical catalyst, the researcher is entitled to conclude that the proposed scheme is valid. But in a large number of reactions it is not possible to isolate the intermediate compounds, either because of their excessive instability, or because they are adhered to the surface of the catalyst. It seems likely that in iron-catalyzed ammonia synthesis, nitrogen molecules are attached to the iron surface.
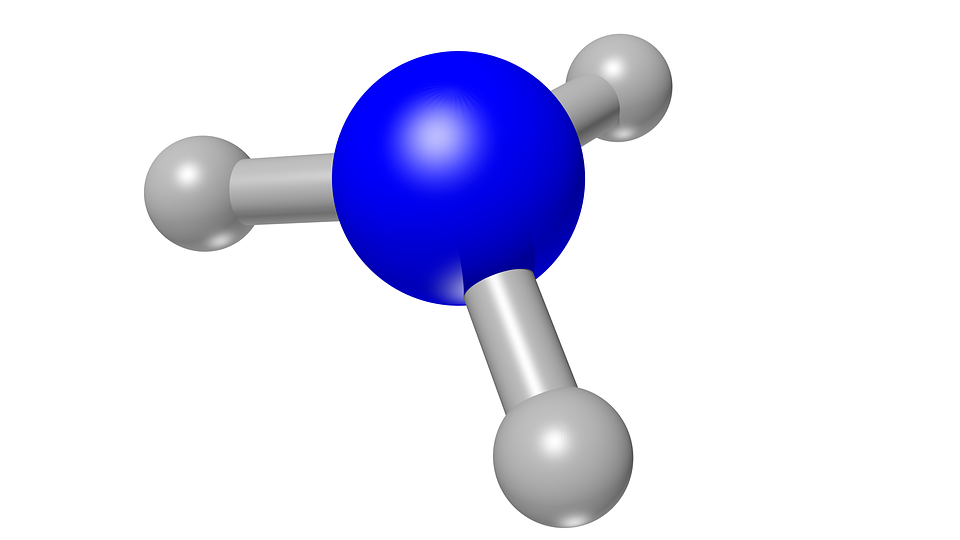
Ammonia molecule. Source: Pixabay
- In some cases, it is possible to discover the intermediate products by implementing a physico-chemical process having sufficient sensitivity to detect the presence of traces of unstable compounds, for example spectrochemical analysis. The intermediate product, to be able to play its role, must not be stable since it is its decomposition which determines the speed of the catalyzed reaction.
Theoretical calculations could make it possible to define the order of magnitude of the bond energies characteristic of the intermediate compounds so that the latter have maximum catalytic efficiency.
The difficulties encountered when trying to isolate unstable intermediate compounds lead to the adoption of the following definition: "any catalyst is a body which can influence the formation of the activated complex". Indeed, whether or not we succeed in isolating the intermediate compounds, we can affirm that the catalyst always intervenes in the reaction by modifying in one way or another the transition state, so that the definition given above is the most general.
- Catalytic reactions are often linked to chain reactions involving radicals. Certain bodies found at the origin of a chain modify their state and are not true catalysts; this is particularly the case of the accelerator of the oxidation of methane to formaldehyde in the presence of nitrogen oxide.
Nitrogen dioxide initiates a chain reaction by forming CH3 radicals:
NO2+ CH4======= CH3+ HNO3
The chain reaction spreads in the following way:
CH3+ O2=======HCHO+ OH
CH4+ OH=======CH3+ H2O
A distinction is usually made between homogeneous catalysis, where the catalyst and the reactants are in the same state of aggregation, and heterogeneous catalysis, where they are in different states of aggregation.
- The field of homogeneous catalysis encompasses the phenomena which take place in solution under the action of acids or bases and which is called acid-base catalysis. The addition of a proton to the most diverse body molecules makes them active by causing a redistribution of the chemical bonds within the molecules and their chemical transformation. Analogous effects are observed when a proton is removed from a molecule.
Let us denote by AH a compound corresponding to the generalized notion of acid and by B a compound corresponding to the generalized notion of base. The evolution of an acid-base catalysis, such as the dehydration of an alcohol, can be described by the following equations: - Case of acid catalysis:
AH+ CH3CH2OH======== CH3CH2OH2(+) + A-
OH2 (Positive Ion)
The unstable particle CH3CH2OH2(+) dissociates into a water molecule and a radical-ion:
CH3CH2OH2(+)======= H20+ CH3CH2
The radical-ion provides an ethylene molecule and the H+ ion:
CH3-CH2+=======H(+) + C2H4
The catalytic effect is therefore reduced to the attachment of a hydrogen ion to a reorganization of the molecule and to the detachment of a hydrogen ion.
An example of a base catalysis is provided by the decomposition reaction of nitramine:
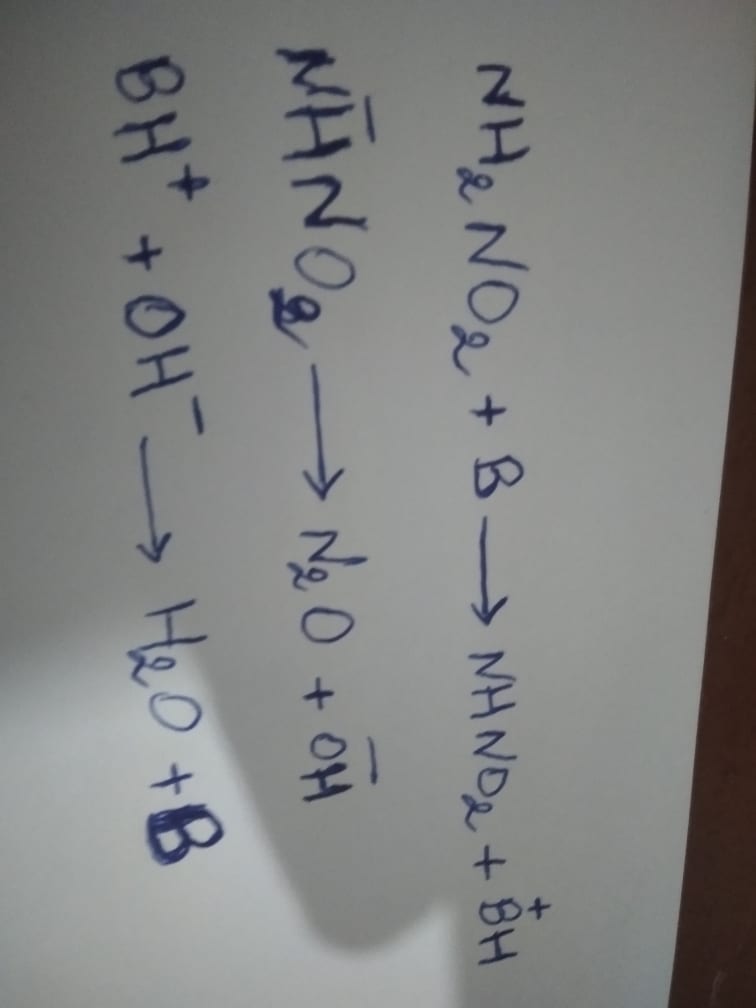
(Self made)
The unstable intermediate product is NHNO2 in the activated state by loss of a proton.
- In a number of homogeneous reactions (oxidation, decomposition of hydrogen peroxide, polymerizations, synthesis of alcohols) a particularly energetic catalytic effect is ensured by complexes of transition metals, particularly those of copper, iron, cobalt, vanadium . To illustrate the mode of action of the complexes, let's examine the scheme of the oxidation of ethylene to aldehyde by catalysis in the presence of palladium salts:
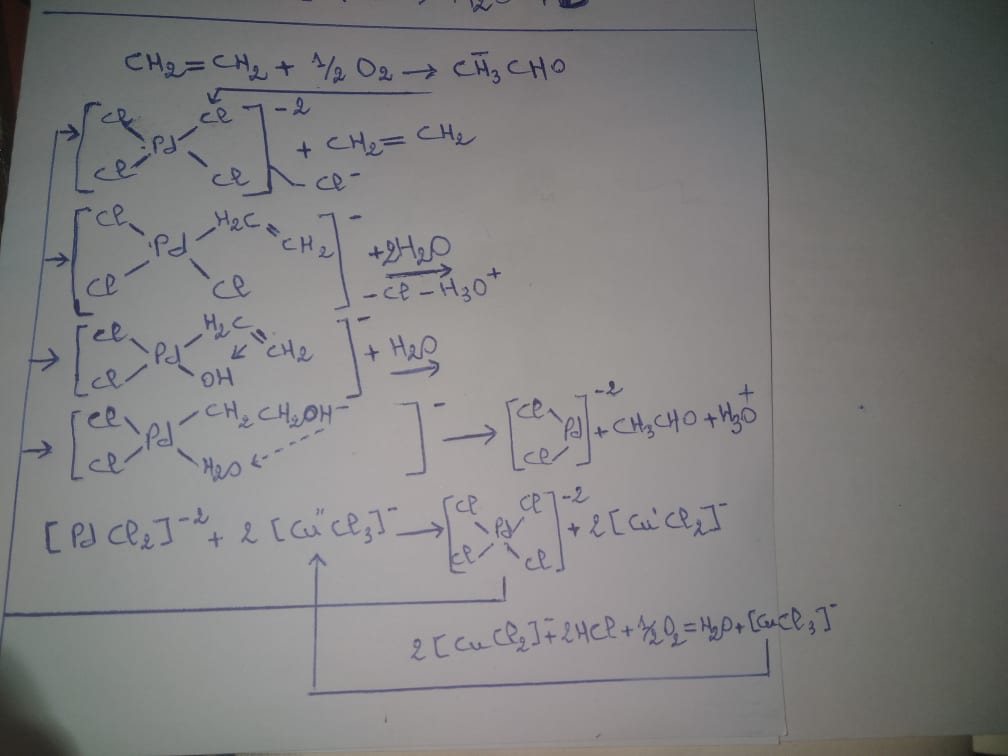
(Self made)
- In natural phenomena the complex catalysts assume the most important functions. Vitamin B12 is a complex cobalt compound, responsible for the maturation of red blood cells; iron metalloporphyrins are components of hemoglobin, catalase and peroxidase, etc.
Red blood cells. Source: Pixabay
It is therefore understandable that the study of the catalytic functions of metal complexes is of particular interest.
References:
[Book: Catalysis From Principles to Applications](Edited by Matthias Belter, Albert Renken, and Rutger van Santen)
Optimization of the Interface Between Catalyst Layer and Proton Exchange Membrane via rolled technique. Sciencegate
Catalysis by SUBHRANGSU DEY. Academia.edu
Types of catalysts. Khan Academy