Among the most significant substances that pollute surface water and must be removed in order to generate drinking water are suspended particles, colloidal solids, and dissolved organic solids. Where solid contaminants are among the most prevalent in surface water. Depending to the size of their particles, two categories of solid pollutants in water may be distinguished, which are:
1- Suspended solids:
It consists of particles that are rapidly accumulated in still water without the use of any chemical reagents and have a diameter greater than 10 to the minus 6 meters.
2- Colloidal solids:
These are called colloids, and they are composed of tiny particles with diameters between 10 power minus 9 meters and 10 power minus 6 meters. Because of its sluggish sedimentation, colloids goes through an agglomeration step before beginning the step of sedimentation.
The term "agglomeration" refers to the collection of small particles by either chemical or physical effect and their transformation into greater sized particles with a sufficient sedimentation period in accordance with the settling time in water treatment basins.
Shared characteristics of suspended and colloidal substances:
-Due to the law of gravity, the particles settle to the bottom of the water basin. The particle diameter, the liquid's viscosity, and the difference between the specific mass of the substance in question and the specific mass of water are three of the most crucial parameters that affect the rate of sedimentation.
-The particles exhibit both adsorption and ion exchange properties.
-Particularly if they are tiny in size and are categorized as colloids with a very slow sedimentation rate, particles are subject to forces other than the earth's gravitational force. Both the ionization and dissociation of the molecules on their surfaces, as well as the adsorption of certain ions by the particles, cause the surfaces of the particles to become charged. This event causes a buildup of particles with opposite charges, which in turn causes the electrical double layer to develop.
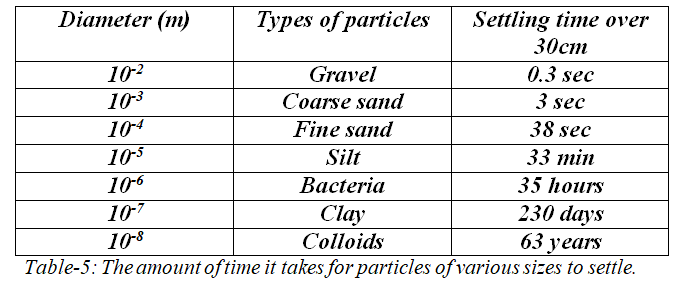
In most cases, the size of the suspended particles have a direct relationship to the sedimentation velocity. Table-5 illustrates how long it takes for different-sized particles to sediment.
[Made using "MS word" and "Paint"]
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.