Entropy is a measure of the disorder of a system. The entropy of a gas is higher than the entropy of a solid, because there are more ways of arranging molecules in a gas, and so there is more disorder. I first made mention of entropy in a post of mine titled ALL ABOUT GASES AS BEING TAUGHT IN THE CLASSROOM.
For a reaction to occur spontaneously there must be an overall increase in entropy. This is the second law of thermodynamics. Thermodynamics is the study of energy transfer. I have already explained the first law of thermodynamics here: It states that energy can neither be created nor destroyed in physical and chemical processes.
One of the most important endothermic reactions for life on this planet is photosynthesis. Although it is endothermic, there must be an increase in entropy or it would not be able to occur at all.
COLLISION THEORY
The collision theory is a model that chemists use to explain how reactions occur. When two molecules react, bonds are broken or made, and there is usually a rearrangement of the atoms. According to collision theory, for this to happen the molecules first have to collide. The collision model was developed from the kinetic-molecular model of gases.
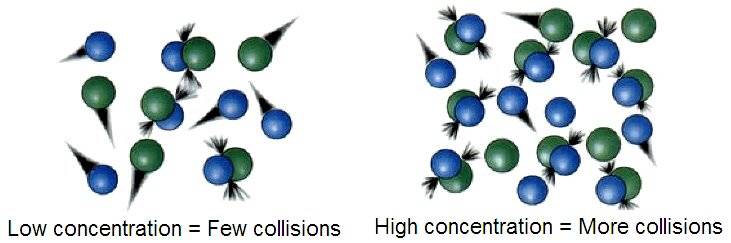
In 1 cm3 of gas at atmospheric pressure and room temperature, it has been calculated that there are about 1027 collisions between two molecules every second. If all these collisions were to lead to reactions, then all reactions that involve gases would be over in microseconds. For example, nitrogen and oxygen molecules, which under normal conditions coexist without reacting, would long ago have reacted to fill the atmosphere with polluting nitrogen oxides. This means that only a certain number of collisions result in reactions. These are called effective collisions and occur when molecules collide with the minimum energy needed to start a reaction, the activation energy.
Talking of the Activation Energy And Stability
Activation energy is sometimes referred to as activation enthalpy. The higher the activation energy for a reaction, the less likely it is to occur. Even though octane looks very unstable compared with carbon dioxide and water, the high energy of activation acts as an energy barrier to the reaction. In petrol, this prevents the reaction from occurring when the tank is filled at the garage, and is the reason why there are ‘No smoking’ signs!
Reaction kinetics is the study of rates of reactions and say petrol is kinetically stable, even though it is energetically unstable. Octane is energetically unstable because it is at a higher energy level than carbon dioxide and water. Sometimes the term thermodynamically unstable is used instead of energetically unstable.
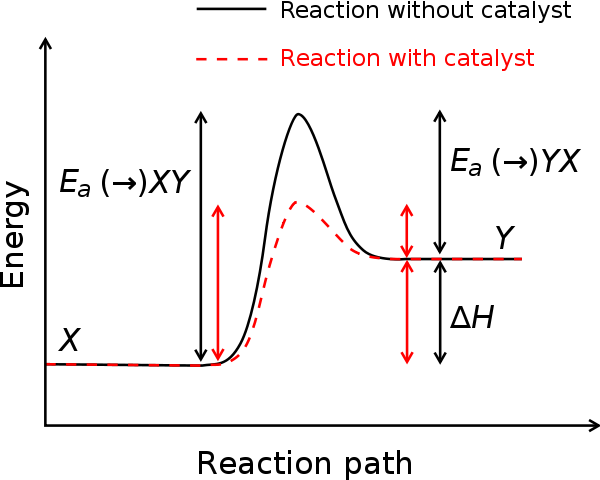
LOWERING THE ENERGY BARRIER: CATALYSIS
In our previous chemistry course we probably learnt that catalysts usually increase the rate of chemical reactions. Catalysts also play a crucial role in the lives of everybody. Protein catalysts called enzymes dramatically increase the rates of thousands of different chemical reactions that take place in our bodies every second. And catalysts are involved in the manufacture of most of the chemicals on which people rely. As a definition: Catalysts are substances that alter the rate of a chemical reaction but can be recovered unchanged at the end of the reaction.
But they do become involved temporarily in the reaction, because: catalysts work by providing an alternative reaction pathway with a lower energy of activation. Catalysts are not put into the overall chemical equation for reactions because they are chemically unchanged at the end of the reaction. While catalysts are not used in the combustion of petrol, they are used in catalytic converters to speed up reactions that control emissions from the exhausts of cars.
Catalysts often work by forming intermediates. In this case the energy profile diagram becomes a little more complicated. The intermediates are of a higher energy than the reactants or products but the overall reaction pathway is still of lower energy.
Sometimes catalysts slow down the rate of a reaction. When they do, they are called negative catalysts or inhibitors. These do not work by raising the activation energy, but instead usually react with molecules produced in the intermediate stages of a reaction and remove them from the reaction pathway.
ANOTHER ENTHALPY CHANGE: THE ENTHALPY CHANGE OF FORMATION
Like the enthalpy change of combustion, the enthalpy change of formation is just one more enthalpy change of reaction. But this time, instead of burning one mole of compound completely in oxygen, we form one mole of compound from its elements.
The standard enthalpy change of formation ΔHꝋf,298is the enthalpy change when one mole of a compound is formed from its elements under standard conditions, that is, 298 K and 100 kPa pressure. ΔHꝋf can be used instead of ΔHꝋf,298 because it’s assumed that the temperature is 298 K unless another temperature is quoted. Standard molar enthalpy change of formation is another name for standard enthalpy change of formation. You will find standard enthalpy changes of formation for compounds in almost any chemistry data book.
The standard enthalpy change of formation of water:
ΔHꝋf (H2O(l)) = -286 kJ mol-1
Applies to this reaction:
H2(g) + ½ O2(g) → H2O(l)
Notice that the value above is also the standard enthalpy change of combustion of hydrogen. The equation for the standard enthalpy change of formation of methanol is:
C(s) + 2H2(g) + ½ O2(g) → CH3OH(l) ΔHꝋf = -239 kJ mol-1
WHY STANDARD ENTHALPY CHANGES OF FORMATION ARE IMPORTANT
You can see the value of knowing enthalpy changes of combustion – because, for example, they allow us to work out the energy we can obtain from a fuel. But why bother with the standard enthalpy changes of formation?
The enthalpy of a substance is its energy content. We cannot measure this and give it an absolute value. It is all the energy associated with the particles in the substance, which includes the nuclei, the electrons and the movements of whole molecules.
We can only measure enthalpy changes, and to do this we must have a reference point. The one that scientists have chosen is the standard enthalpy change of formation. It applies to any compound, while for elements it is zero. It is a very important piece of information about a compound, and once you know the value for each of the different substances in a reaction, you can calculate the enthalpy change for that whole reaction.
However, before this is considered, we must look at how we obtain values for the standard enthalpy change of formation. Carbon exists in two forms, diamond and graphite. The most stable of these forms is graphite, which is the standard state used for standard enthalpy changes of formation. Sometimes you will see Cgraphite written in the equation.
MEASURING THE STANDARD ENTHALPY CHANGE OF FORMATION
Some standard enthalpy changes of formation can be measured experimentally. For example, we can measure ΔHꝋf of carbon dioxide by using a bomb calorimeter and combusting graphite. The ΔHꝋf of magnesium oxide can be measured in the same way.
When a compound is easily synthesized from its elements under normal conditions, we can usually measure ΔHꝋf directly. However, many standard enthalpy changes of formation cannot be measured directly, which is where we need an indirect approach using energy cycles.

THE INDIRECT METHOD FOR CALCULATING ENTHALPY CHANGES OF FORMATION USING ENERGY CYCLES
This method relies on Hess’s law, which states that if a change can be brought about by more than one route, then the overall enthalpy change for each route must be the same provided that the starting and finishing conditions are the same for each route.
This is conservation of energy again – energy cannot be created or destroyed in chemical reactions, so the energy changes for a reaction must be the same, whether it takes place in one step or in a whole series of steps.
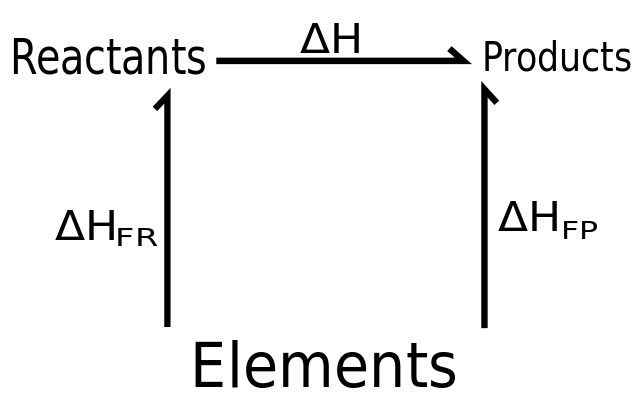
Methane cannot be prepared directly from its elements, but we can use Hess’s law to calculate its standard enthalpy change of formation. By burning carbon, hydrogen and methane, we can measure their standard enthalpy changes of combustion directly. We can therefore devise an energy cycle with two routes in it, as shown. By Hess’s law: The overall enthalpy change for route 1 = the enthalpy change for route 2.
So:
ΔH1 + ΔH3 = ΔH2
ΔH1 is what we are trying to find, in this case the standard enthalpy change of formation of methane, ΔHꝋf (CH4(g)).
For route 1:
ΔH2 is ΔHꝋc for carbon (graphite) = -393 kJ mol-1
Plus 2 × ΔHꝋc for hydrogen = 2 × -286 kJ mol-1
(Remember: ΔHꝋc for hydrogen is for burning one mole of hydrogen. In this energy cycle there are two moles of hydrogen.)
ΔH3 = standard enthalpy change of combustion of methane, ΔHꝋc (CH4(g)) = -890 kJ mol-1.
So:
ΔH1 = ΔH2 - ΔH3 = -393 + 2(-286) – (-890)
ΔHꝋf (CH4(g)) = -75 kJ mol-1
EXAMPLE
Calculate the standard enthalpy change of formation of methanol, given that:
ΔHꝋc (CH3OH(l)) = -726 kJ mol-1
ΔHꝋc (C(s)) = -393 kJ mol-1
ΔHꝋc (H2(g) = -286 kJ mol-1
ANSWER
Step 1. Write out the enthalpy change you have been asked to calculate – in this case ΔHꝋf (CH3OH(l)):
C(s) + 2H2(g) + ½ O2(g) → CH3OH(l)
Step 2. Construct an energy cycle with two alternative routes. It helps if you always put the enthalpy change you want to calculate along the top of the cycle and call it ΔH1.
Step 3. Write out the enthalpy changes for the two routes:
ΔH1 + ΔH3 = ΔH2
Step 4. Decide what enthalpy changes are represented by ΔH1, ΔH2 and ΔH3. It is a good idea to do this on the energy cycle diagram first.
ΔH1 = ΔHꝋf (CH3OH(l))
that is, the enthalpy change we wish to calculate.
ΔH2 = ΔHꝋc (C(s)) + [2 × ΔHꝋc (H2(g))]
ΔH3 = ΔHꝋc (CH3OH(l))
Step 5. Rearrange the equation in Step 3 and insert the values of the enthalpy changes that are known:
ΔH1 = ΔH2 - ΔH3
ΔHꝋf (CH3OH(l)) = ΔHꝋc (C(s)) + [2 × ΔHꝋc (H2(g))] - ΔHꝋc (CH3OH(l))
ΔHꝋf (CH3OH(l)) = -393 + 2(-286) – (-726) = -239kJmol-1
Thus the standard enthalpy change of formation of methanol is -239 kJ mol-1
Thank you for reading.
REFERENCES
https://en.wikipedia.org/wiki/Collision_theory
https://www.britannica.com/science/collision-theory-chemistry
https://www.khanacademy.org/science/high-school-biology/hs-energy-and-transport/hs-enzymes/a/activation-energy
https://lavelle.chem.ucla.edu/forum/viewtopic.php?t=3639
https://www.researchgate.net/post/What_is_the_relationship_between_activation_energy_and_thermal_stability#:~:text=Thermal%20stability%20relates%20to%20how,something%20in%20an%20Arrhenius%20form.
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/14%3A_Chemical_Kinetics/14.07%3A_Catalysis
http://www2.ucdsb.on.ca/tiss/stretton/CHEM2/rate06.htm
http://www1.lsbu.ac.uk/water/enztech/mechan.html#:~:text=The%20lower%20the%20potential%20energy,uncatalysed%20reaction%20(Figure%201.1).
https://www.sciencedirect.com/topics/chemistry/enthalpy-of-formation
https://courses.lumenlearning.com/boundless-chemistry/chapter/standard-enthalpy-of-formation-and-reaction/
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Map%3A_Physical_Chemistry_for_the_Biosciences_(Chang)/03%3A_The_First_Law_of_Thermodynamics/3.6%3A_Thermochemistry
https://en.wikipedia.org/wiki/Standard_enthalpy_of_formation#:~:text=The%20standard%20enthalpy%20of%20formation%20is%20measured%20in%20units%20of,per%20mass%20or%20amount%20guideline).
http://www.docbrown.info/page07/delta1Hc.htm
https://www.chemguide.co.uk/physical/energetics/sums.html
https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/07%3A_Thermochemistry/7.7%3A_Indirect_Determination_of_%CE%94H_-_Hess's_Law
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app
and including @stemsocial as a beneficiary, which give you stronger support.