Chlorine and the other halogens have an affinity for hydrogen. Chlorine reacts with compounds that contain hydrogen to form hydrogen chloride, or hydrochloric acid when the reaction is carried out in aqueous solution. Chlorine behaves as an oxidising agent.
Reaction of chlorine with water
Chlorine reacts with water to form an acidic solution that is a mixture of two acids, hydrochloric acid and chloric(I) acid. This is an example of a disproportionation reaction, in which chlorine is both oxidised and reduced during the reaction:
Cl2(aq) + H2O(l) → HCl(aq) + HOCI(aq)
This reaction is the basis of one of the chemical tests for chlorine: namely, it turns moist blue litmus paper first red and then white. Chlorine reacts with the moisture to form the acidic solution which turns the litmus paper red. The chloric(I) acid acts as a bleach and turns the paper white.

Reaction of chlorine with cold dilute alkali
Cold aqueous sodium hydroxide can react with chlorine to produce chlorate(I) ions, ClO-, and chloride ions, Cl-:
Cl2(g) + 2OH-(aq) → CIO-(aq) + Cl-(aq) + H2O(l)
Cl2(g) + 2NaOH(aq) → NaClO(aq) + NaCl(aq) + H2O(l)
A solution of aqueous sodium chlorate(I) is the liquid bleach found in most homes.
Reaction of chlorine with hot concentrated alkali
When chlorine is added to hot concentrated aqueous sodium hydroxide, the overall reaction is:
3Cl2(g) + 6OH-(aq) → ClO3-(aq) + 5Cl-(aq) + 3H2O(l)
This reaction occurs because of the disproportionation of chlorate(I) ions. Chlorate(I) ions lose electrons to give chlorate(V) ions, and gain electrons to form chloride ions:
3ClO-(aq) → ClO3-(aq) + 2Cl-(aq)
Sodium chlorate(V), NaClO3, is used as a weedkiller. It is also reduced to form ClO2, chlorine dioxide, which is a safer bleach than elemental chlorine for paper and textiles, though it is less efficient.
Oxidation of iron(II) ions
Chlorine oxidises aqueous iron(II) chloride to form aqueous iron(III) chloride:
CI2(g) + 2Fe2+(aq) → 2Fe3+(aq) + 2Cl-(aq)
Reaction of halogens with aqueous sodium thiosulfate
Aqueous chlorine and aqueous bromine oxidise aqueous thiosulfate ions into sulfate ions:
4Cl2(aq) + S2O32-(aq) + 5H2O(l) → 10H+(aq) + 8CI-(aq) + 2SO42-(aq)
Iodine is a less powerful oxidising agent than either chlorine or bromine, and so the reaction does not give sulfate ions. Instead, it gives S4O62-(aq):
I2(aq) + 2S2O32-(aq) → 2I-(aq) + S4O62-(aq)
Chemical test for an oxidising agent
One chemical test for an oxidising agent uses aqueous potassium iodide. An oxidising agent liberates iodine from aqueous potassium iodide, and the amount of iodine can be determined using volumetric analysis with a titration against aqueous sodium thiosulfate. The presence of iodine is shown by a blue-black coloration with starch solution.
COVALENT HALIDES
All non-metal halides are covalent and have a simple molecular structure. Typically, non-metal halides are liquids at room temperature with a low boiling point. Some metals will also form covalent halides, which is the case when the oxidation state of the metal is +3 or above. In such cases the metal ion that could be formed, e.g, Al3+, has a very small ionic radius and is highly charged, and consequently distorts the electron cloud around the anion so much that the resulting bond has sufficient covalent character to be considered as being covalent.
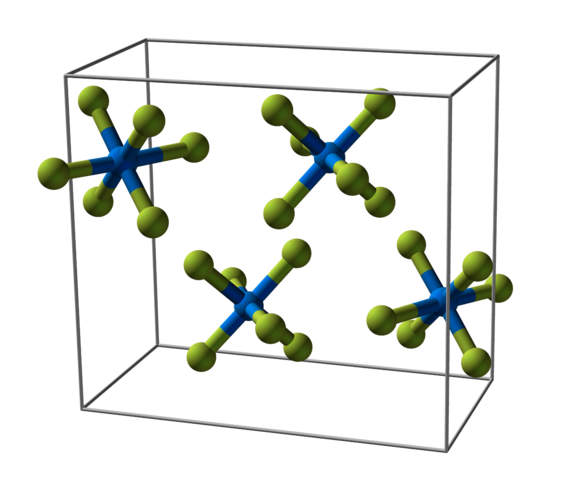
HYDROLYSIS OF COVALENT HALIDES
Almost all the covalent halides can be hydrolysed to give an acidic solution. It is a typical property of covalently bonded chlorides that they can be hydrolysed to form hydrochloric acid or hydrogen chloride if only a limited supply of water is available. One exception to this rule is tetrachloromethane which does not react with water at all.
The hydrolysis of covalent halides, such as phosphorus(III) bromide and phosphorus(III) iodide, provides a suitable way to prepare hydrogen bromide and hydrogen iodide.
HYDROGEN HALIDES
All the hydrogen halides have the formula HX, where X is F, CI, Br, I or At. They all are colourless acidic gases that are highly soluble in water, in which they form an acidic solution. The bonding in a hydrogen halide is covalent, but the molecule is polar with its halogen end being slightly negative and its hydrogen end slightly positive.
NOBLE GAS COMPOUNDS
Until 1962, chemists believed that the noble gases, such as xenon, did not react. Since then, several compounds that contain xenon and fluorine have been prepared. Xenon difluoride, for example, is formed when excess xenon is heated with fluorine gas at 400 C:
Xe(g) + F2(g) → XeF2(g)
Xenon difluoride forms colourless crystals, which are stable at room temperature in a dry atmosphere. It is quite surprising that this compound should be so stable. Xenon has a stable octet of electrons, so by reacting with fluorine and attaining an oxidation number of +2 in the difluoride, it should lose its stability.
By changing the mole ratios of xenon to fluorine, it is also possible to prepare xenon tetrafluoride, XeF4, and xenon hexafluoride, XeF6. All the xenon fluorides are very powerful oxidising agents, since xenon in a positive oxidation state is less stable than elemental xenon with an oxidation number of 0. Xenon difluoride oxidises water to form xenon, hydrofluoric acid and oxygen:
2XeF2(s) + 2H2O(l) → 2Xe(g) + 4HF(aq) + O2(g)
REACTION OF HALOGENS WITH HYDROGEN
The halogens react with hydrogen to give hydrogen halides, but the rate of reaction decreases with increasing atomic (proton) number of the halogen. The reaction between hydrogen and fluorine takes place very rapidly, even at low temperatures, whereas the reaction between hydrogen and iodine is reversible and needs elevated temperatures:
H2(g) + F2(g) → 2HF(g)
When heated, hydrogen reacts with chlorine, and when a mixture of the two gases is subjected to ultraviolet light the reaction can be explosive.
Thermal decomposition of hydrogen halides
The thermal stability of the hydrogen halides decreases with increasing relative molecular mass, in a similar manner to the decrease in the bond energy from H – F to H – I. In the decomposition of hydrogen iodide, it is better to describe the reaction as an equilibrium process:
2HI(g) ⇌ H2(g) + I2(g) ΔH is positive
Le Chatelier’s principle states that the position of equilibrium of a system shifts to minimise the effect of any change in the external conditions such as increasing the temperature or the pressure. In the equilibrium process above, pressure has no effect whatsoever, because there is no volume change during the reaction – the number of moles of gas is the same on both sides of the equation. Since the decomposition is endothermic, an increase in temperature favours the formation of the two elements, because the reaction from left to right absorbs energy.
CHEMICAL TEST FOR HYDROGEN HALIDES
Hydrogen halides are colourless acidic gases that are able to react with bases such as ammonia gas. This reaction is interesting in that two gases react together to make a white solid dispersed in a gas, correctly described as a smoke. This can be used as a chemical test for the hydrogen halides:
NH3(g) + HCl(g) → NH4Cl(s)
AQUEOUS SOLUTIONS OF THE HYDROGEN HALIDES
All the hydrogen halides dissolve in water to form acidic solutions. Hydrogen chloride forms hydrochloric acid, hydrogen bromide forms hydrobromic acid and hydrogen iodide forms hydroiodic acid:
HX(g) + H2O(l) → H3O+(aq) + X-(aq) where X is Cl, Br or I.
The acid strength increases from hydrochloric acid to hydroiodic acid. This is because the H – I bond is weaker than the H – Cl bond. The bonding changes from covalent in the hydrogen halide to ionic in the corresponding acid.
All three acids are strong and display the typical reactions of strong acids
- They form hydrogen with metals above hydrogen in the electrochemical series.
- They form carbon dioxide with carbonates and hydrogencarbonates.
- They form salts with bases, such as metal oxides and hydroxides.
- They fully dissociate to form aqueous hydrogen ions.
Concentrated hydrochloric, hydrobromic and hydroiodic acids contain a large percentage of water, since if an attempt is made to concentrate them further, the gaseous hydrogen halide is evolved.
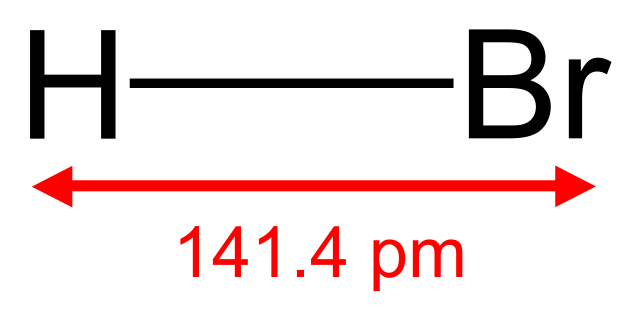
Oxidation of hydrohalic acids
Hydrochloric acid, hydrobromic acid and hydroiodic acid can all be oxidised to form the elemental halogen. The reaction involves the removal of hydrogen and increase in the oxidation number of the halogen atom. For example:
PbO2(s) + 4HCl(aq) → PbCl2(aq) + 2H2O) + Cl2(g)
The ease of oxidation increases as the atomic (proton) number of the halogen increases. So it is quite easy to reduce hydroiodic acid, but much more difficult to reduce hydrochloric acid. Hydrochloric acid can be oxidised by lead(IV) oxide, manganese(IV) oxide or potassium manganate (VII) to make chlorine, but less powerful oxidising agents can be used to convert hydrobromic acid and hydroiodic acid into bromine and iodine, respectively. In other words, the reducing power decreases from hydroiodic to hydrochloric acid.
I will like to pause here for now and continue in my next post.
Thanks for coming
REFERENCES
http://www.chemistryexplained.com/elements/A-C/Chlorine.html
https://www.britannica.com/science/chlorine/Physical-and-chemical-properties
https://www.sciencedirect.com/science/article/pii/S0043135407005003
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites_
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Book%3A_Inorganic_Chemistry_
https://en.wikipedia.org/wiki/Noble_gas_compound
https://www.sciencedirect.com/topics/chemistry/hydrogen-halide
https://en.wikipedia.org/wiki/Hydrogen_halide#:~:text=Hydrogen%20halides%20are%20diatomic%20inorganic,Compound
https://onlinelibrary.wiley.com/doi/pdf/10.1002/kin.550060104
https://www.bbc.co.uk/bitesize/guides/zg337p3/revision/2
https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites.
https://www.sciencedirect.com/science/article/pii/S0069804008702954/pdf?md5=1cf06c9d57b07895410bff614cbc88ad&pid=1-s2.0-S0069804008702954-main.pdf&_valck=1
https://opentextbc.ca/chemistry/chapter/18-11-occurrence-preparation-and-properties-of-halogens/
https://pubs.acs.org/doi/10.1021/ed078p116
https://www.researchgate.net/publication/314064308_By_Hydrohalic_Acids
Hello,
Thank you for this discussion. I tried to follow the chemical interactions, and sort of got lost. I was interested in finding out why halogen bulbs release UV rays. Anyone who is sensitive to UV discovers this rather quickly (true also for fluorescent bulbs). Perhaps this question is not relevant to your post. If so...sorry :) (I only took one course in high school chemistry--which I enjoyed--many, many years ago.)
Hi, @agmoore. Can you tell me where you missed the post, I might come in. Sorry about that though.
Regarding your question about halogen bulbs. Halogen lamp or bulb is a type of lamp that emits light when heated. It contains a bulb that's filled with a noble gas mixed with halogen (most especially iodine or bromine) under very high pressure. It emits UV because it requires a very high temperature to function. Halogen lamp works at a very high temperature, so it produce more UV light, just like the sun. I hope you know that the sun too emits UV light and it's even more intense now due to the depletion of the ozone layer that's meant to shield the excess UV light from the sun, as a result of our incessant burning of fuels and release of harmful chemicals into the atmosphere.
So, basically, halogen lamp emits UV and can cause skin cancer and some other harmful effect because it requires a whole lot of heat to function. But the harmful effects can be reduced through the use of special casing to cover the lamp.
Thank you for coming.
Hello @empressteemah,
Thank for that thoughtful response. I am kind of a human barometer for UV. The sun, fluorescent lamps, halogens--exposure to all of these (any UV source) is harmful for me. However, LEDs seem to be fine. So I guess LEDs do not contain a noble gas that has to be super-heated.
And, yes, I notice a well-screened bulb is much more tolerable than one that is unscreened.
Thank you again for that informative reply.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.