Author: @madridbg, via Power Point 2010, using public domain images. Unknown
Greetings and welcome dear readers of this beautiful platform, especially to all those subscribers who make daily life in the @Stemsocial community. The topic that concerns us this time is of a scientific nature and is related to the use of chiral molecules and their implication in metabolic functioning.
In this sense, it is necessary to remember that the conceptual approach related to chiral molecules was addressed in this community in one of the discussions that usually take place and that allows the acquisition of diverse knowledge in terms of the development of science, so we will review and retrospectively review the functions of chirality in drugs and how these chemical aspects are related to the functioning of molecules.
CHIRALITY IN CONCEPTUAL TERMS

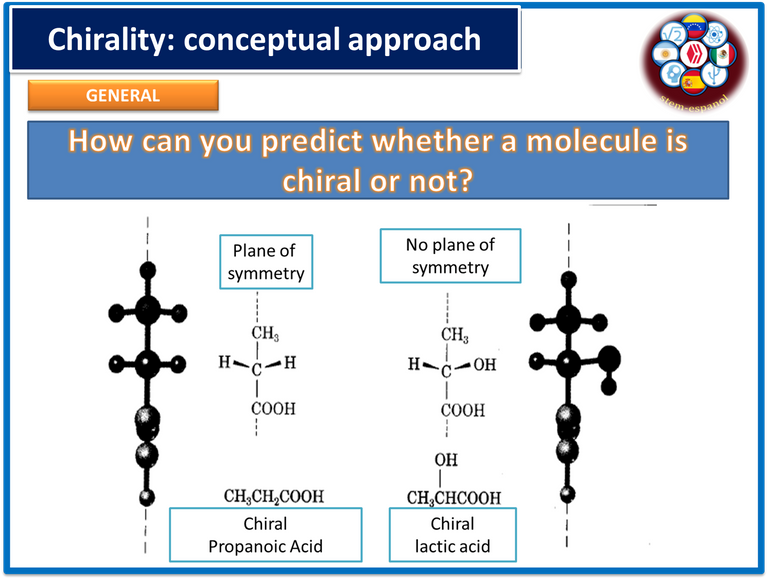
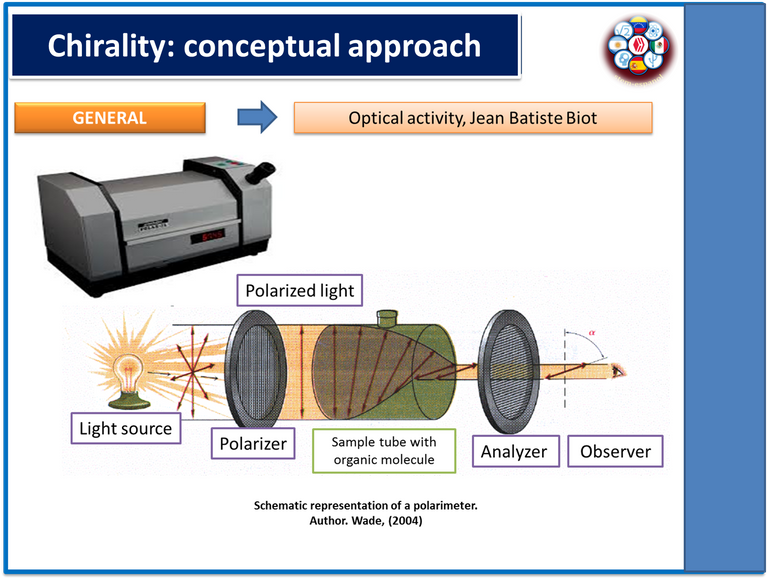
As is well known, the term chirality refers to the spatial organization adopted by organic molecules and their ability to deflect the plane of polarized light, either to the left in the case of levorotatory compounds or to the right in the case of dextrorotatory compounds.
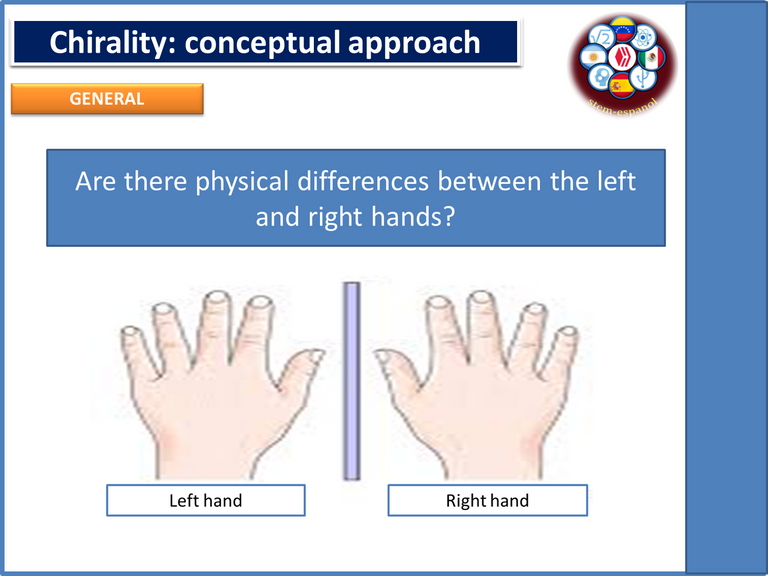
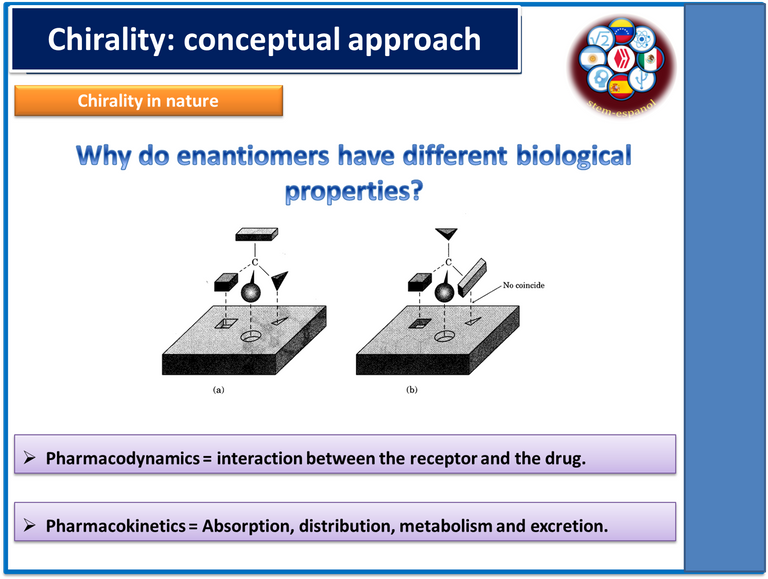
So in order to understand in a visual way the aspects related to chirality, we can use in an analogous way the functioning of our hands, since if we place our left hand on the right and vice versa we can observe that one of the hands is the mirror image of the other and consequently they do not have the ability to be superimposed, in other words, one image is the reflection of the other as if they were looking in a mirror, behavior that in chemical terms is known as chirality.
In this sense, this type of behavior is glimpsed in organic compounds and according to the spatial organization they adopt will influence their biological activity and consequently the pharmacodynamic and pharmacokinetic process of these varies, so that chirality is a highly relevant property since at the pharmacological level the formation of enantiomeric compounds can occur, racemic mixtures, diastereoisomers, among others, and each of these compounds can fulfill different functions at the metabolic level and in many cases can cause adverse effects as in the case of thalidomide and the teratogenic effects that this compound generated in pregnant women.
Author: @madridbg, via Power Point 2010, using public domain images..
Therefore, it is to be expected that chirality is assumed as a characteristic of matter, which is determinant in the symmetry of biological structures at different scales, whether at the level of DNA or of the different tissues that form the muscles in our body, without excluding the cardiac, so that at the scientific level it is assumed that nature is asymmetrical and consequently we will observe different behaviors towards the right or left band that conforms it.
In such a way, living matter understood as amino acids, carbohydrates, sugars, lipids, among others, are chiral which means that they are formed by enantiomers or exactly identical compounds that are formed by the same amount of atoms but whose properties and behavior is different, simply by the fact of turning in different planes the polarized light, a determining property in the optical activity and pharmacological action that the compounds may present.
CONTROLLED SYNTHESIS OF CHIRAL MOLECULES

Previously it was thought that chirality was a property that was only required through living matter and that it was the only one that had the ability to generate it, however, through the evolution and progress of science, it has been possible to establish conscious mechanisms of action that allow to obtain enantiomers under different organizations or spatial arrangements (S and R) either levogiro or dextrógiro, which are important and decisive in the manufacturing processes of drugs, pesticides, flavoring substances, sweeteners and other chemical compounds, knowing that each enantiomer obtained has a specific activity and function that is different from its mirror image.
It should also be noted that in many cases of the enantiomers obtained, only one of these is the one that presents a functional optical activity, while the other lacks utility or, failing that, the utility may be toxic.
In this sense, at the laboratory level, several strategies are currently being developed to control the sign of chirality, in other words, to obtain the compound or enantiomer that the analyst requires, this is achieved since it has been possible to develop techniques based on selective enantiomerism, which is associated with the use of pure enantiomers of natural origin as a mechanism of synthesis.
Author: @madridbg, via Power Point 2010, using public domain images.
This process is carried out through the use of chiral auxiliaries that help the specific substrates to react selectively and obtain enantiomerically pure products. Finally, through a process called asymmetric catalysis, the mechanism to achieve the asymmetric synthesis of the desired enantiomer is oriented, a process that is seen as the best scenario to obtain optically active compounds.
We must take into account that in the different processes used, the different enantiomers will always be obtained depending on the starting compound and through selective chirality what is done is to look for a conscious mechanism that allows obtaining the desired enantiomer at the end of the process, knowing that the yield will generally be 50% since the remaining enantiomer remains as residue within the system, so that in this selective chirality purification steps are applied through chiral catalysts that allow obtaining and generating efficiency on the quantities of the desired and obtained compound.
CHIRALITY AND THE PROCESS OF TRANSFERS FROM THE MACRO TO THE MICRO SCALE

Having understood the conceptual bases about chirality and to respond to this section of the subject, it is necessary to rely on recent publications carried out through the Faculty of Chemistry of the Institute of Theoretical and Computational Chemistry of the University of Barcelona, in this space of disclosure, researchers have managed to generate a mechanism that allows transferring chirality from the macroscale to the microscale, that is, from the molecules of the compounds to the nanoparticles of a given substance.
This process allows to control the signs of chirality of the enantiomer obtained, which in the opinion of the researchers is an inventive that behaves as a self-assembly at nanometric scale, thus modulating the geometry of the helical reactor and consequently allows the obtaining of optically active compounds according to our preference.
Author: @madridbg, via Power Point 2010, using public domain images.
By means of this technique what is executed is a process of transferring the optical activity from the macro scale to obtain optically active nanoparticles, so that for the process to work, variables such as fluid dynamics, mass transport, as well as the positioning of the reaction zones in the spiral region of the reactor must be controlled.
Thus, by controlling the aforementioned variables in the helical reactor, it is possible to control the asymmetry of the secondary flows, obtaining that the concentrations of the reagents are exposed to one of the two sides associated with the chirality, extracting at the end of the process the enantiomer from the side that presents greater optical activity depending on the type of metabolic process or work that we wish to execute, which makes this mechanism of action much more effective and selective for obtaining optically active compounds or nanoparticles.
Author: @madridbg, via Power Point 2010, using public domain images.
In this sense, the work in general opens new perspectives and a more holistic vision about the process of enantiomeric selectivity at the molecular level, for which the helical reactor allows leaving aside the enantiomeric compounds of high purity and it would be enough only with the combination of the geometry and the operating conditions in the equipment to obtain the desired compound, which represents a great advance in obtaining new drugs at a lower cost that facilitates the life of people.
FINAL CONSIDERATIONS

Undoubtedly, the chirality process is an important chemical behavior in the biological functioning and in the pharmacodynamics and pharmacokinetics of drug preparation, being able to develop a mechanism of action to obtain compounds selectively is a substantial advance since through the evolution of computational chemistry it has been possible to determine the functioning and behavior of the different compounds at macro and microscale and how they will affect the treatment of diseases and the metabolic functioning.
Hence, understanding the chirality process generates greater effectiveness in enantioselective synthesis and consequently in obtaining new substances that fulfill specific optical activities.
BIBLIOGRAPHY CONSULTED

[1] Chang, R. (2010). Chemistry. Tenth edition. McGraw-hill Interamericana publishers. ISBN: 978-607-15-0307-7.
[2] Juaristi Eusebio. (2005). Left and right in chemistry: chirality. Science Artículo: Acceso en línea .
[3] McMURRY E., John y Fay C., Robert. (2008). General Chemistry. Fifth edition PEARSON EDUCACIÓN, Mexico, 2009 ISBN: 978-970-26 1286-5.
[4] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). GENERAL CHEMISTRY. Eighth edition. PEARSON EDUCACIÓN. S.A., Madrid.
[5] Vaquero Juan José. Chemical methodologies in the discovery of new drugs. Universities of Alcalá, Complutense and S. Pablo-CEU Interuniversity Doctorate in MEDICAL CHEMISTRY. Article: Online access .
OF INTEREST

For more information related to the areas of science, technology, engineering and mathematics, feel free to visit #stemsocial and #stem-espanol, communities that promote scientific advances in these areas.

Thanks for this very interesting blog. I enjoyed reading it and I didn't know about this connection with chirality and molecules. I am happy to have learned something today.
In fact, here I could not prevent myself from smiling. Chirality is a well known concept in particle physics (we say that matter is chiral). Of course, this is slightly different from what you discuss here. But only slightly! We have left-handed elementary particles and right-handed elementary particles that behave very differently.
Cheers!
Greetings @lemouth first of all thank you for taking the time to stop by, read and value my publication. With respect to chirality, without a doubt, nature presents us with a lot of diversity and it is these variables that cause science to marvel at the discovery of new processes that allow us to improve our quality of life.
In the past, chirality in drugs caused serious effects that are still suffered today, a clear example is the teratogenic effects caused by thalidomide, however, with scientific advances, the pharmacokinetics and pharmacodynamics of any substance that is destined to fulfill a metabolic function are evaluated, where the study on the optical activity is essential for drugs to be approved, the surprising thing is that the compounds despite having the same amount and type of atoms, perform different functions, product of the spatial arrangement they adopt.
Thanks for your valuable comment. Regards
Thanks for the extra interesting details about drug acceptance. There are definitely useful at least for someone like me who do not know much about this.
Cheers!
Great article!
!1UP
Thank you @kwskicky for the rating and support for my writing. Regards
You have received a 1UP from @kwskicky!
@stem-curator, @neoxag-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
Thank you @curation-cartel for the support shown.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thank you @stemsocial for your support and appreciation of my publication. Regards