
Image courtesy of: Fulvio Ciccolo
Greetings and welcome dear readers, the subject of science is constantly evolving, a process that allows us to generate a diverse field of knowledge to meet the current demands of our society.
In this sense, through the following publication, we will address relevant aspects associated with electrophoresis and how through the principles of isoelectric components we can develop a crucial technique in chemical and microbiological analysis.
So we can establish that when a solution is subjected to an electric field, we can determine that the solute molecules with a net positive charge, move towards the cathode, while the molecules with a net negative charge move towards the anode, at this point the displacement generated is known as electrophoresis.
Consequently, it is to be expected that there are internal factors that exert an action on the kinetic velocity of the molecules, hence we can find that there is a force exerted by the electric field on the particles, accompanied by the charge of these particles.
Therefore, at a mathematical level we can establish a direct relationship between the charge of the particle (q) and the force of the electric field (E), likewise, we can establish the existence of a friction force of the system or environment on the particles (F) that directly affects the velocity (V), which allows us to establish the following equation.

Similarly, it is necessary to understand that the friction coefficient depends on the size and shape of the particle, so that large or symmetrical molecules have greater resistance in terms of friction than smaller or compact molecules, hence their friction coefficient is higher, however, when the electric field is activated, the molecules undergo rapid acceleration until they reach a point of equilibrium in terms of speed of movement, a process that we represent in the previous equation.
Based on the above principles, it is necessary to understand that we can find two types of electrophoresis, which respond to a methodology on paper and the other is executed on gel as a support medium. The first one is usually used to separate mixtures of small molecules that have undergone a loading process. Throughout the process, a filter paper previously moistened with a buffer solution is used to control the pH inside the containers containing the electrodes.
Likewise, a drop of the sample under study is placed on the paper and the electric current is applied for periods of time that depend on the analyst, although many studies usually establish as a principle 2 hours to achieve the separation of the components, after which the paper is removed, dye is applied seeking to dye the components to identify the distances that will be subjected to specific analysis.
It is necessary to understand throughout the process that the mixture will be displaced either to the anode or cathode according to the charge it presents and the path is another variable of analysis that allows to establish chemical principles according to the properties of the unknown substances, so that they are comparable with patterns previously stored in the database.
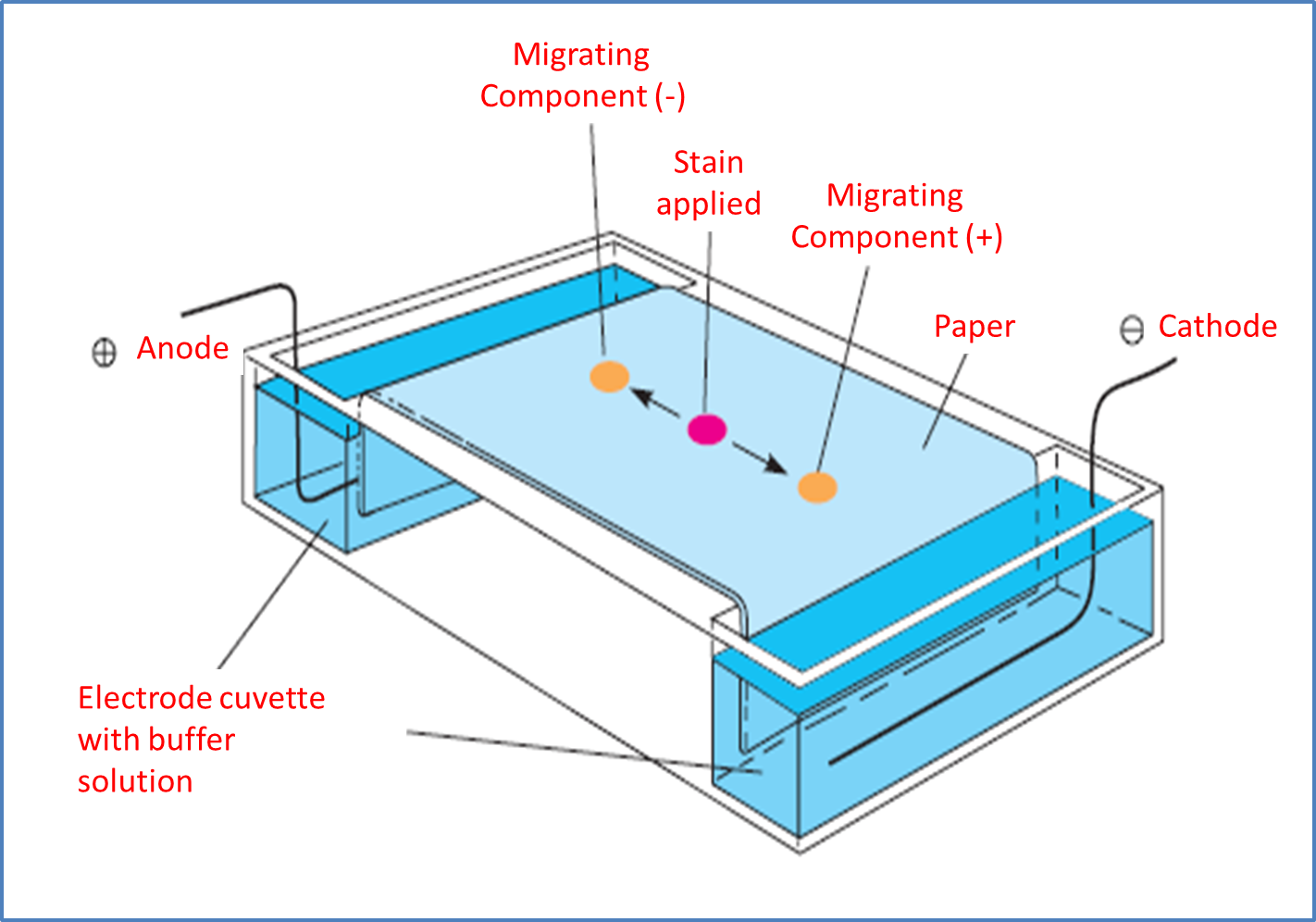
Image adapted from MATHEWS, C. K.; VAN HOLDE, K. E.; AHERN, K. G.
Gel electrophoresis, as a technique, is most useful for protein analysis and for identifying nucleic acids, which is why it is the most important technique in molecular biology. It starts by placing the gel in the middle of a buffer solution located between two glass plates.
Methodologically, the materials generally used are polyacrylamine, as a cross-linked polymer, and water-soluble agarose. In this sense, the plate is placed between the electrode compartments, selecting the lower part as the anode or cathode, depending on whether anions or cations are to be separated.
Next, with the help of a pipette or a graduated instrument, we proceed to place the amount of sample that we will analyze previously in solution, in the same way a sample of localization formed by glycerol and water soluble dye is added, at this point, chemically the localization solution becomes denser because of the glycerol and does not mix with the buffer solution present in the upper electrode chamber.
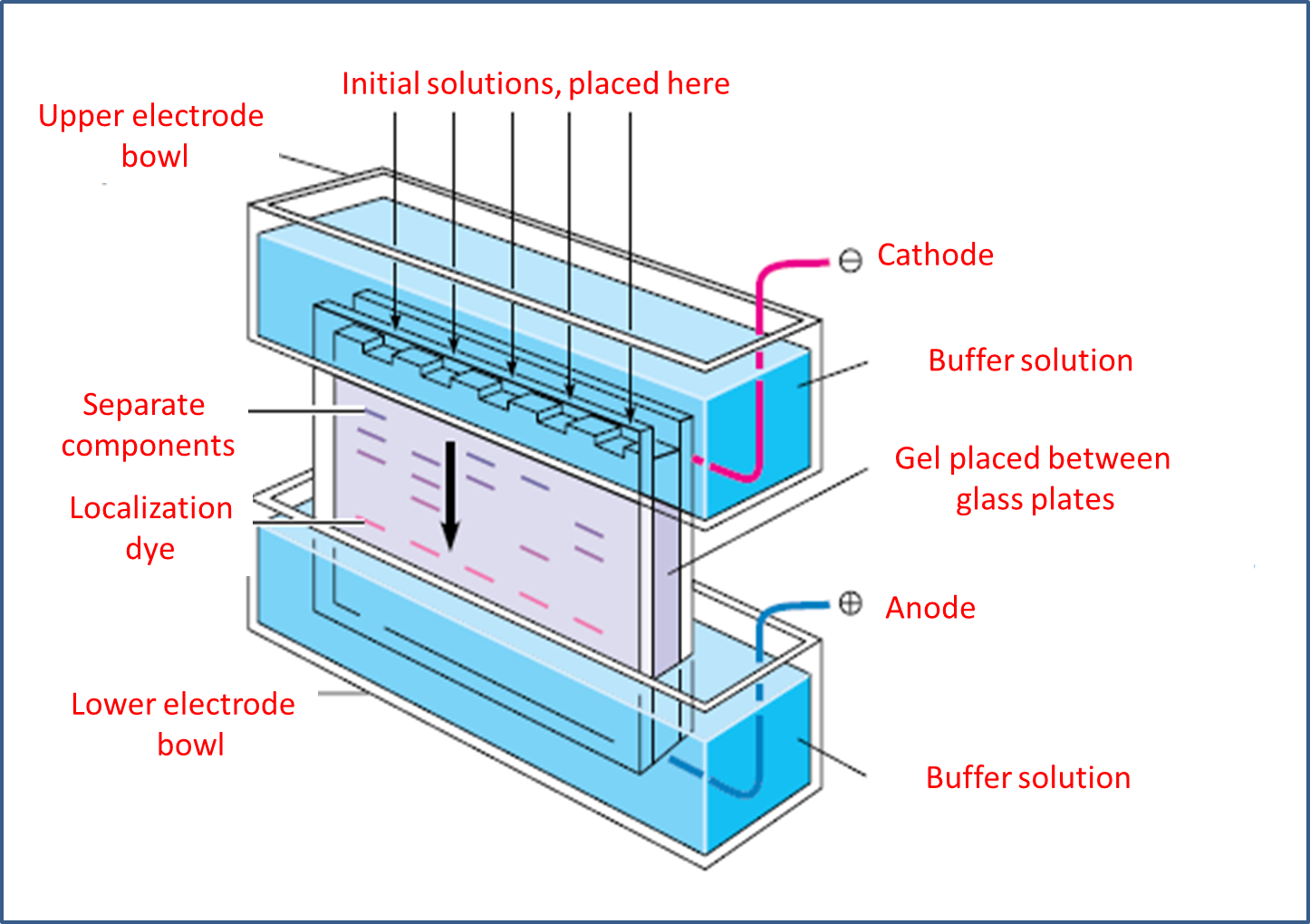
Image adapted from MATHEWS, C. K.; VAN HOLDE, K. E.; AHERN, K. G.
For its part, the dye separates and moves faster than most macro-ions, which makes it possible to follow the evolution stages of the process or experiment with ease. Regarding the current, it is kept on until the dye is sufficiently displaced and is at the bottom, from there, the gel is removed from between the plates and usually stained with a specific dye that cradles proteins and nucleic acids. Finally, a photograph of the plate is taken and the information is saved since the protein or nucleic acid mixture was applied as a narrow band at the top of the gel.
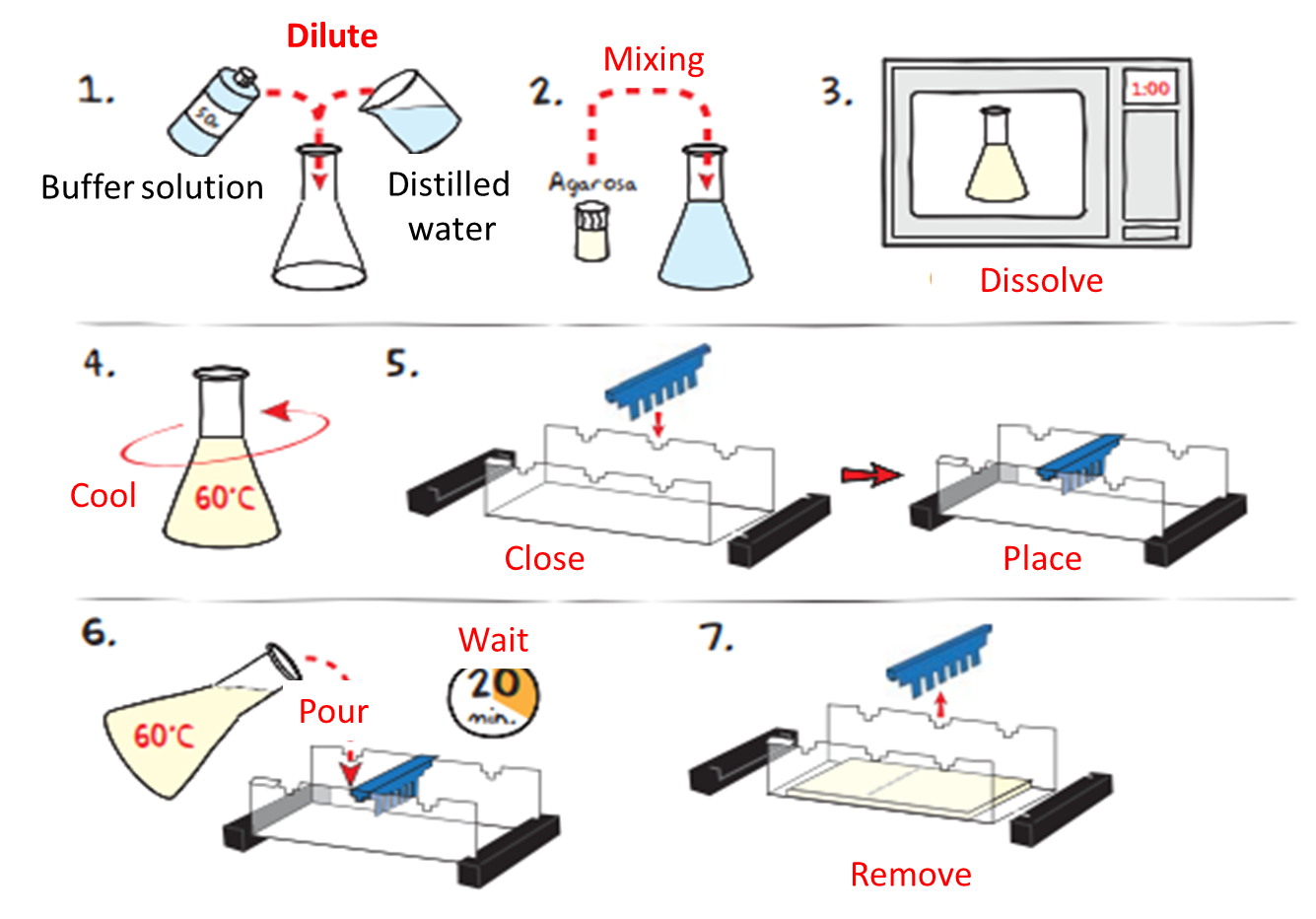
Image adapted from: EDVOTEK
As we can see, through the implementation of the scientific method and experimentation, it has been possible to establish procedures that have allowed the scientific evolution and practical development of the same.
BIBLIOGRAPHY CONSULTED

[1] Carmen Alicia Padilla Peña and collaborators. Electrophoresis of nucleic acids in agarose gels. Isolation and electrophoretic electrophoretic characterization of plasmid DNA. Article: Online Access
[2] EDVOTEK. Principles and practice of agarose gel electrophoresis. Article: Online Access
OF INTEREST

1. The cover image was made by @madridbg, used public domain image.
2. For more information related to the areas of science, technology, engineering and mathematics, feel free to visit #stemsocial and #stem-espanol, communities that promote scientific advances in these areas.
