There are many ways to get around the locked door. You can break the door, or you can break the lock, but nothing matches the skill of the key maker. You can just summon the key maker show him the lock and ask him to design a key that fits perfectly into the grooves of the lock. Well, such is the beauty of the adaptive immune system.

Recap
In the previous episode, we learnt about the very initial stages of the viral infection. We saw how the infected cells call for help and warn it's neighbours and resident immune cells of the imminent threat. In response to these signals, the neighbouring cells turn on genes to slow down the spread and replication of the virus. The neighbouring cells also join to call for help. Soon after the resident innate immune cells call for reinforcements from the circulation.
We looked at the various mechanisms by which cells from the innate immune system neutralize the virus. While some cells kill the virus there are others like the natural killer cells which identify and kill the stressed and the infected cells.
Nevertheless, we also learnt that these responses are non-specific. The innate immune system acts via recognizing general patterns and cues. Imagine, living in a neighbourhood where you know there is a lot of criminal activity. Now you have cops who are good at responding to suspected criminal activity and they tend to broadly know of the operating gangs. They try their best to minimize the crime but also end up causing unwanted damage. This is where the cells of the adaptive immune system step in.
The adaptive immune system
As the name suggests, the adaptive immune system 1 can adapt. The adaptive immune system has a superpower to design the molecules that bind specifically and exclusively to only a particular foreign molecule. This helps them to recognize and tag the infectious agent and infected cells specifically for destruction and also minimise the collateral damage to a major extent. There are two major types of cells in the adaptive immune system - The B cells and the T cells 2.
Both B and T cells are made in the bone marrow. Each B and T cell is born with a unique receptor that can bind to a particular molecule. If they bind to the self molecule they commit suicide. Those that do not, lurk around for few days in the dark allies of the lymph nodes. If they do find a foreign molecule in the allotted time, they are chosen to carry forward their lineage and mount an immune response. If they don't, they are lost into oblivion.
The B cells
The B cells are a bit nerdy. They create these molecular drones which flow throughout the body and specifically bind to molecules of the pathogens they are designed for. These drones called antibodies tag the pathogen and the cells infected by them. This makes it easy for other immune cells, and for the molecules of the complement system to find and kill the infectious agent.
B cell development
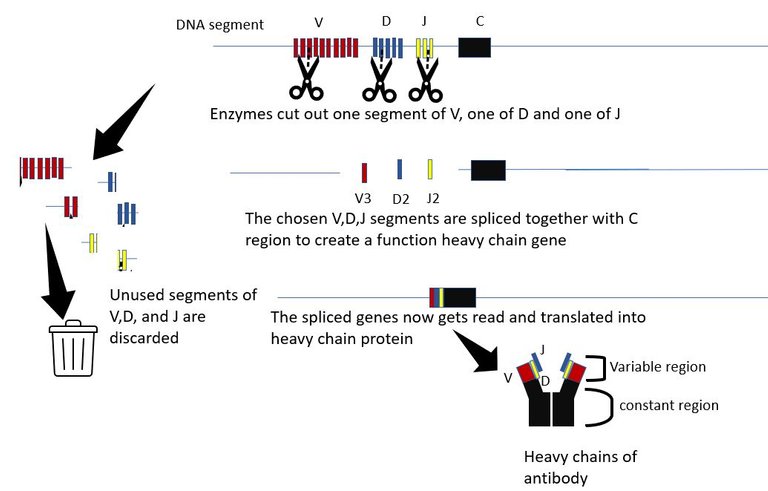
In the core of the bone marrow, every day 109 B cells are produced. And, each B cell that is made is unique. That is each B cell makes an antibody with unique specificity. For this, in the pre-B cells reorganize their chromosomal sequence for genes that encodes the B cell receptor (BCR). A BCR is nothing membrane-bound antibody. Each antibody is complied by joining 7 different genes (4 different genes for the heavy chain of the antibody and 3 different genes for the light chain). In our chromosome, we have multiple versions of each of these 7 genes. Each cell picks up a different set of these and recombines them with different variations to make a unique functional B cell receptor (See Box 1 at the end of the article) 3. Once, this a done a naïve B cell is ready.
What's the purpose of life?
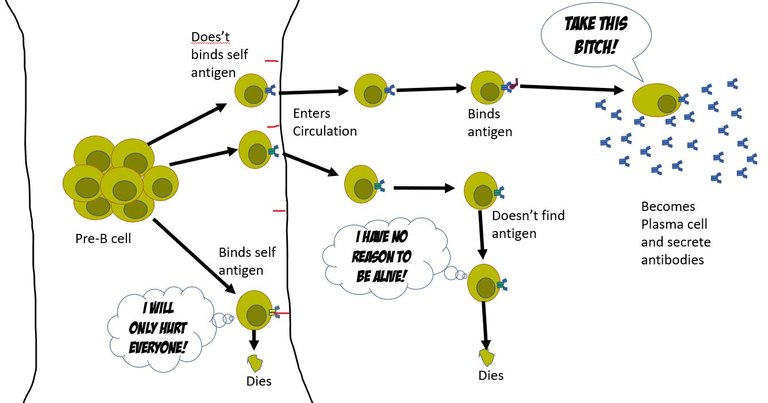
At this point, the naive B cell has no clue what its unique BCR would bind to, or even if it would bind to anything at all. However, if BCR binds to anything in the bone marrow, it's assumed that it binds to a self- molecule. And hence such a naïve B cell is instructed to self destruct. The other naïve B cells that don't bind to self-antigens are set free to enter the circulation and find a damn purpose in their life. Well, usually only 1 out of 10^5 of these B cells ever find a purpose (a foreign antigen).
The antigen-presenting cells of the innate immune system pick up the pieces of viral products from the site of infection. They meet the naïve B cells in lymph nodes. They present the antigens they have been carrying (pieces of the virus) on their surface and let the naïve B cells try their luck to bind to them. If they do bind to the antigen the B cells are primed for activation and transformation into the antibody-secreting cells. For this, the B cell changes the tail region of the heavy chain from hydrophobic to hydrophilic. The antibody with a hydrophilic tail doesn't get inserted into the membrane, and hence gets secreted out 3.
Illustrated by @scienceblocks
The red tape
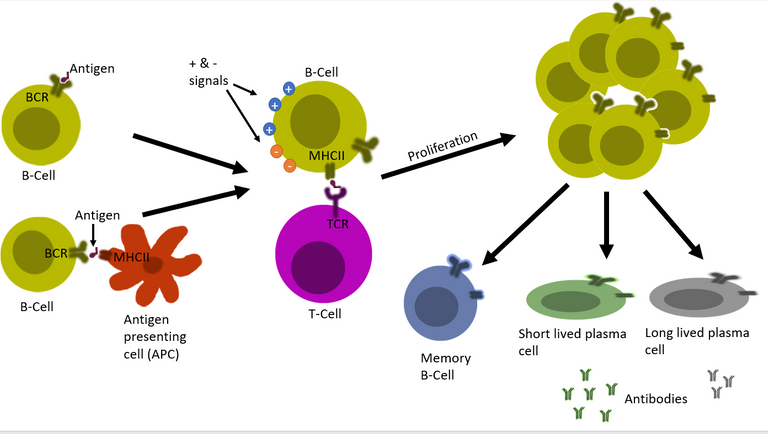
But, it's not an on and off switch. Just binding to the antigen doesn't turn a naïve B cell into antibody-producing plasma cells. The body has evolved many positive and negative inputs for this process. This is to ensure that antibody that attack self molecules are not produced 4, 5, 6, 7. It depends on the nature of the infecting pathogen, the load of the pathogen, cytokines present on the inflammatory milieu, and also on the presence of T helper cells against that pathogen. Only under the correct context where positive inputs outnumber the negative inputs the B cell gets permission to proliferate and then transform into antibody-producing plasma cells.
Illustrated by @scienceblocks
B cells optimize and improvise
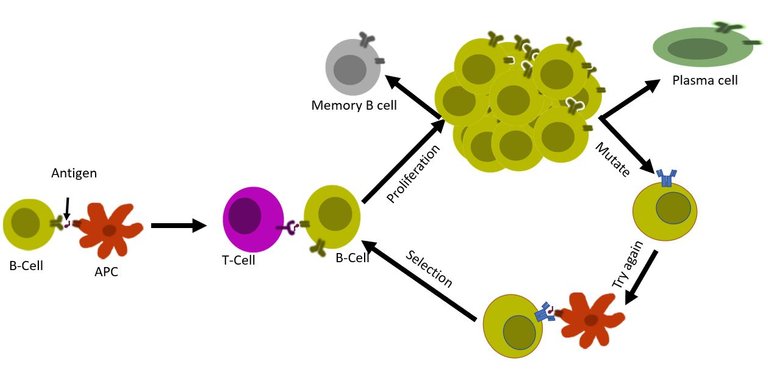
First, a set of B cells that binds to antigen interacts with the T cells. If the signal is green then it is decided whether the B cell will undergo further mutations in the genes for the B cell receptor (a process known as somatic hypermutation). This B cell with the mutated receptor is then tested again for its binding affinity. So not only a B cell is capable of producing unique antibodies, but it also is capable of editing its genome to optimize this antibody. Once the system is satisfied that the B cell now is matured and perfect for producing specific antibodies with strong affinity, it allows the B cell to transform into plasma cell 8, 9, 10.
Illustrated by @scienceblocks
How do antibodies fight the virus?
The plasma cell then produce antibodies that can fight off the infection in one of the following ways -
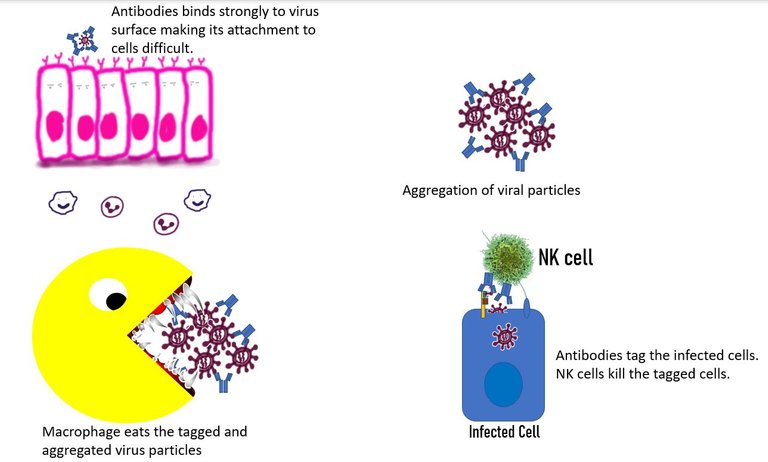
Neutralization of the virus. This happens when the antibody binds to the receptor-binding domain of the virus and prevents its interaction with the vulnerable cells 11.
Agglutination of the virus. Antibodies can cause the viruses to stick together. The large antibody virus complexes reduce the efficiency of the virus to be freely available to infect cells or travel distances. Furthermore, these complexes can then be eaten up by phagocytes such as macrophages or can be attacked by the complement system. 12
Antibody-mediated phagocytosis. The antibody bound to the virus, binds to the Fc receptor on the surface of cells of the innate immune system. It can then be internalized and eaten up. While this mechanism is cool it is also of concern. When a non-neutralizing antibody causes internalization of the virus in the immune cells, it can lead to viral infection of the immune cell. Even if the virus doesn't replicate in the immune cell, it can lead to the increased immune response by these cells which can add to disease severity. This process known as antibody-dependent enhancement is well documented for the Dengue virus. 12
Tagging the infected cells Not only can antibodies tag the virus itself, but they can also tag the infected cell that has virus proteins on its surface. Once tagged, this cell can be easily and specifically targeted by the natural killer cells. 13
The B cell remembers
Anyhow, the antibody-producing plasma cells and antibodies usually last long enough to fight the infection and then they start declining slowly. I mean these plasma cells hang around for a while, just in case the virus returns and then they die. But that's just the story of short-lived plasma cells (SLPCs). There is another variety of plasma cells called long-lived plasma cells (LLPCs). LLPCs stick around in specialized niches in bone marrow, where they depended on positive signals from the niche to live for decades. They keep producing antibodies, in case the virus returns back 5-10 years later 9.
If it does, it is unlikely that this virus will be able to do much harm and many times won't even be able to infect the person (depending on the nature of the antibody present).
Whether LLPCs are formed or not depends on how the whole scene plays out and on their successful migration to pro-survival niches.
But, SLPCs and LLPCs are not the only fates of the activated B cells. Our body also believes in keeping a record of an undifferentiated B cell that has seen the antigen. This cell also known as the memory B cell lasts for decades. If the virus were to try to reinfect the same person, they will be there to respond.
Well, they won't nip the infection at the bud, but the body won't have to start from scratch. You don't have to wait for the right naïve B cell to bind to an antigen. Instead memory B cells on identifying the similar pathogen molecule will just start dividing and differentiating into plasma cells to produce antibodies.
Also note that some memory B cells are picked up early on during the infection, from the naïve B cells with lower antigen-binding strength. The benefit for having a low-affinity memory B cell sitting there is that during the reinfection even if the virus has changed a little, they can still weakly bind to it9. Post binding they can always enter the process of affinity maturation to generate plasma cells with strong binding affinity.
The T cells
Unlike B cells that secrete specific antibodies molecules in the environment to detect pathogens, T-cell depends on the direct cell to cell contact. This is why T cell immunity is referred to as cellular immunity. If B cells are officers who make drones and robots to capture and kill the enemy. Then T cell would be officers who go on the ground and search each and every household for the enemy.
Illustrated by @scienceblocks
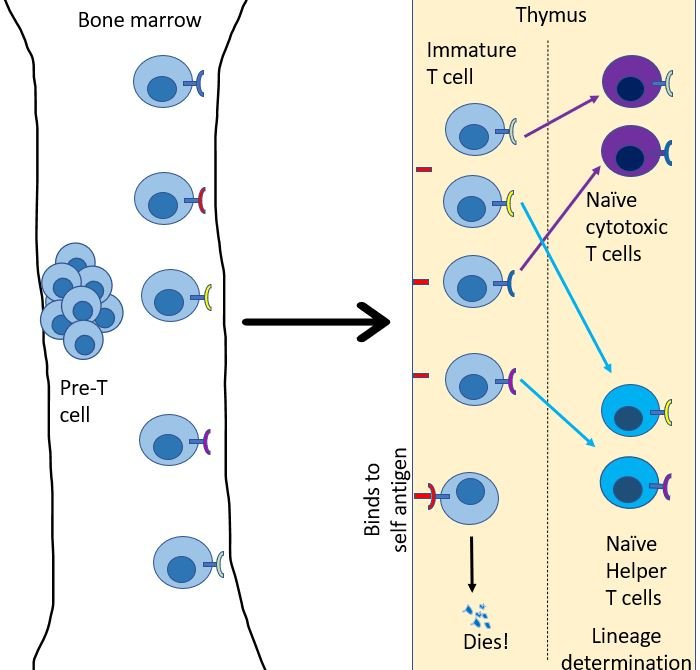
Just like B cells, T cells start their journey in bone marrow, but as immature naïve T cells13. Every day, many immature T cells are created in the bone marrow. From here they migrate to go reside in the thymus. Each of these immature T cells develops a unique version of the T cell receptor (TCR). Just like BCRs, TCR's are created by randomly editing gene products to generate a unique sequence that will bind to only a particular antigen molecule14. Then, their fate is decided in the thymus. Any T cell that binds to a self-antigen, dies. Then they choose their lineage. They either chose to be a helper T cell or a cytotoxic T cell.
T helper cells help!
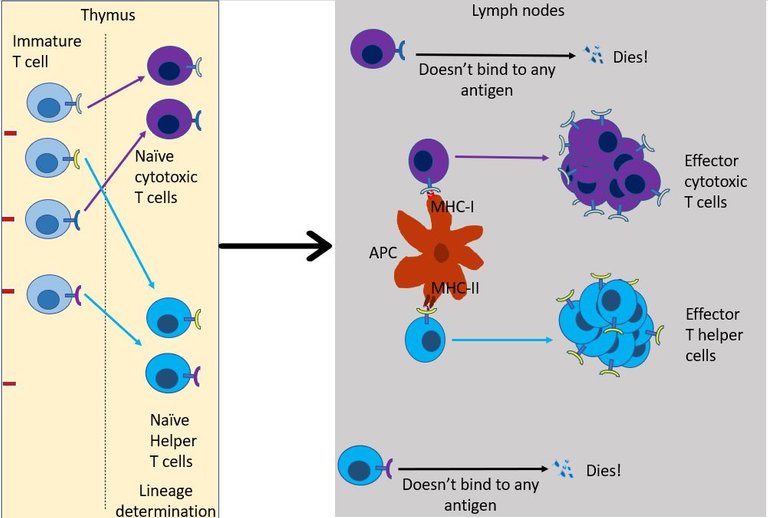
As the name suggests the role of T helper cell is to help 15. They scan the antigens being presented by the innate immune cells. If TCR of the naïve helper T cell bind to antigen-presenting cells it multiples and expands its colony, And, it also transforms into an effector T helper cell. But this is provided right co-simulation and cytokines are present in the environment16. Otherwise, the default response of T cell when it binds to antigen is to become unresponsive and develop tolerance to that molecule 17. This is again important to avoid the development of self-reactive T cells.
Illustrated by @scienceblocks
If everything goes right then, the effector T helper may then chose to do one of the following18 -
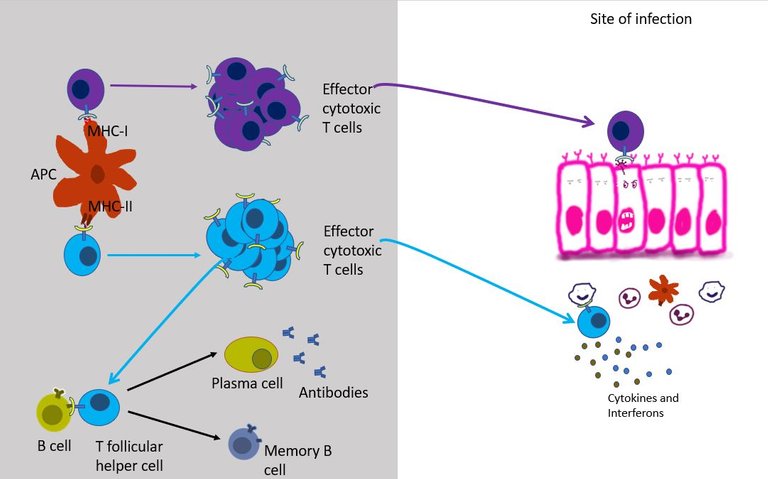
It can differentiate into T follicular helper cells and stay in the lymph node follicles and help B cells survive, grow and make antibodies (see above).
It can migrate to the site of infection, where it joins the other cells in secreting more cytokines. Over here it stimulates other innate immune cells such as macrophages and neutrophils to kill the virus by increasing their phagocytic activity or by increasing Reactive oxygen species (ROS) production by these cells. The interferons secreted by effector T helper cells also stimulates NK cells to come and kill the infected cells.
But most importantly, the T helper cell produces signals that aid the recruitment and development of effector cytotoxic T cells.
Illustrated by @scienceblocks
Cytotoxic T cells kill!
Well, unlike the helper T cell which only reads the MHC class II of innate immune, cytotoxic T cells can read MHC class I present on the surface of all cells in the body. This gives cytotoxic T cells unique authority to check residents of every household in its neighbourhood. If TCR of an effector cytotoxic T cell binds to the infected cell, it immediately starts the program to kill that cell 19. Once bound it can kill the infected cells just like NK cells (see the previous episode) - that is either by poking holes in the cells and injecting toxic proteins in it or by simply asking the cell to commit suicide.
The T cell memory
Well, the show goes on until the viral infection has been cleared. After that most of the effector CD4 and CD8 T cells die. But a small pool of the effector T cells is kept aside to differentiate into memory T cells, which can live in there for decades in the best case scenario20. If the virus does happen to return the memory T cells respond rapidly.
However, will the T cell memory form? How long will it last depends on a lot of factors ranging from the nature of the pathogen involved, duration of infection, the load of the pathogen, strength of interaction between dendritic cells and T cells, and the cytokines stimulating the T cells? For instance, common cold viruses hardly induce T cell memory. Then the long duration of infection, such as with HIV can be detrimental for T cell memory. Both insufficient and overexposure to antigen cause impaired memory. It is thought that memory T cells form during the contraction phase (when the infection is resolving), hence in prolonged infection T cells might simply get exhausted. High concentrations of cytokines such as IL2 in the inflammatory microenvironment favours the formation of effector cells over memory cells21.
To sum it up the adaptive immune system differs from the innate immune system in mounting targeted responses and keeping a memory of its targets. Innate immune system response is like airstrikes by a fighter jet dropping bombs in enemy territory. And adaptive immune system response is - targeted attacks carried out by drones and ground forces. It identifies the face of the enemy and destroy it. It also keeps the biometric details of the enemy are kept on the archives, in case it is ever need again. But is our enemy, so passive? Sure the virus too must have tricks up its sleeves. While those tricks are what we are going to discuss in the next episode, it would be interesting if anyone could take a guess. Meanwhile don't forget to check the bonus box below, which explains how B cells (and similarly T cells) generate the ability to bind those trillions of possible pathogen molecules out there. Also, do leave your questions in the comments.Summary

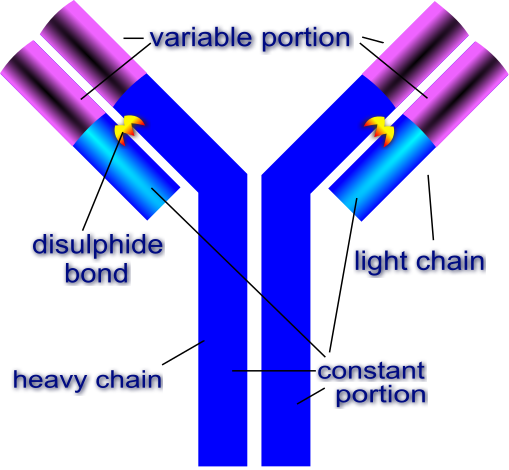
General structure of the antibodyAn antibody macromolecule is made of 2 heavy chains and 2 light chains. Each heavy and light chain has a constant region and a variable region. The constant region is responsible for interacting with other cells and molecules of the immune system. The variable part is what binds to the foreign antigen. Source: Image by Di
gitalShuttermonkey | CC BY-SA 3.0
The generation of antibody diversity The variable part is what binds to molecules of the invader. And as the name suggests it is variable and each B cell is born with a unique set of heavy and light chain variable parts. The variable region of the heavy chain is encoded by 3 genes, The V gene, the D gene and the J gene. The are multiple copies of each of the V, D and J genes in the chromosome, organized sequentially in a file. Each copy differs slightly from the other. The proteins in the body which are molecular scissors, cut out these sequences at random. All but 1 V gene, 1 D gene and one J gene are cut out and discarded. The randomly picked V, D and J genes are then spliced together along with the constant region. Hence, if there are n V genes, m D genes and k J genes copies in that DNA segment, the total number of different versions of heavy chain that can be created is n x m x k. For humans, there an about 51 V segments, 27 D segments and 6 J segments. Hence, 51x27x6=8262 heavy chains can be made just by picking segments for these genes at random. Similarly, about 316 different light chain molecules can be made by similar permutation and combination. This gives us 8262x316 = 2.6 million antibody molecules with unique specificities.
And it doesn't end there. The cuts and splicing of these segments are not perfect. Extra DNA bases are often removed and added during the process. These irregular junctional cuts, joining and editing add 3x107 more possibilities. This increases the number of possible antibody specificities to an order of 1014 - 1014. And that's not all. B cell can further diversify and optimize the antibody it makes, by introducing random mutations in the variable gene regions during the process of affinity maturation. 3, 22.
The generation of diversity for the T cell receptor is not very different. T cell receptor consists of an alpha chain and a beta chain. Both these chains have a constant region and variable region. The variable region of the beta chain has options to pick from 52 Vs, 2 Ds, and 13 Js. Similarly, the variable region of the Beta chain has options of 70 Vs and 61 Js. Hence, (52x2x13)x(70x61) gives us 5.8 million possible TCRs. Add to this the shenanigans that happen at the junctions. It is estimated that approx 2x1011 possible junctional variations can be created in cutting, splicing and editing of TCR genes. Hence, the estimate of total number of TCRs that can be created anybody is estimated to be somewhere between 1017 to 101822.
And, do not forget we carry two copies of these genes on two chromosomes. Though one cell picks only one of the two chromosomes to pull this off. So the number of antibodies and TCRs that can exist in a person is actually double our estimates.


References
- The Adaptive Immune System
- Generation of lymphocytes in bone marrow and thymus
- The Generation of Antibody Diversity
- IMMUNOREGULATION, TOLERANCE, AND THERAPEUTIC IMMUNOLOGY
- Interleukin-24 inhibits the plasma cell differentiation program in human germinal centre B cells
- B-cell tolerance and autoimmunity
- Mechanisms of central tolerance for B cells
- Regulatory mechanisms of B cell responses and the implication in B cell-related diseases
- Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination
- Strategies to guide the antibody affinity maturation process
- Functional and Protective Role of Neutralizing Antibodies (NAbs) Against Viral Infections
- Immune responses to viruses
- Functions of natural killer cells
- Mechanics of T cell receptor gene rearrangement
- Helper T Cells and Lymphocyte Activation
- T-cell activation
- Tolerance and Exhaustion: Defining Mechanisms of T cell Dysfunction
- Helper T Cells and Lymphocyte Activation
- T cell-mediated cytotoxicity
- Effector and memory T-cell differentiation: implications for vaccine development
- T cell responses: naïve to memory and everything in between
- T-cell receptor gene rearrangement
You can reach out to me on discord if you are in stemsocial discord. Or you can even send me a DM. My discord handle is the same as my hive - @scienceblocks. You can also ping me on my telegram handle: @UncertainHeisenberg. Or follow me on Twitter: @scienceblocks1

Great article, don't understand everything but nevertheless still a good read
Hi. Sorry for those parts. If possible, do let me know which parts were not clear. I can explain them and also take care of making it better in future.
browsing through hive and I saw this post. Can't believe that there is such informative blogs out here I taught all of the hive blog is about splinterlands haha. Enjoyed the read.
Ah well thanks for this comment 🙂. I really appreciate your kind words. Hope to keep sharing more such articles in the future. @stemsocial (or previously known as @steemstem) community has many good informative articles of you want to have a look. Also, @stemgeeks is yet another community where good STEM articles are shared.
Written in such a creative way. Enjoyed it.
The organs and systems that work inside our body work in a very complex way, and no one can know how complicated our body parts work in order to survive, so I think we must thank God at every moment for what we are now and for the good health that we have.
It's great that you likened it to a lock and key because that is a perfect analogy to what happens in the immune systems inside our bodies.
Many viruses enter our bodies and we are always exposed to infection with any virus by picking it up from any person we meet, especially in these bad days with the presence of the Covid 19 virus, so everyone should take care of himself to protect himself, his family and those he loves from these deadly viruses.
Thank you for this valuable information you provided us in this blog.
I still remember about the video update, it's just there's a lot on my plate these days.
Soon.(tm) ;-)
I will wait. 🙂
Congratulations @scienceblocks! Your post has been a top performer on the Hive blockchain and you have been rewarded with the following badge:
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPQue molleja de palabrería como dicen los maracuchos, trate de entender pero no soy médico ni nada que se le parezca.