Catalyst and Chemical Industries
In the recent times, catalyst has become a saving grace in making the earth more habitable by turning the climate killing gas CO2 to group of more useful product.
Natural Forces and it's effect on the earth environment
For almost 5 billions years that the earth has been existing , the available
factors contributing to changes in the earth environment has been natural forces such as volcanic eruptions which release gases such as water vapour, CO2, SO2, H2S and some times hydrogen halides. Another natural force is ocean current. Ocean current transfer warm water from the equator where the ocean act as heat retainer to the remaining water body outside the equator . How does this happen?. The Ocean simply retain heat that would have gone back to the space after the sun has set. So the retained heat is transfered to the rest of the globe through a continuous flow of ocean water as a result of the impact of surface wind, earth rotation, temperature and some other factors on the ocean water. Through evaporation, heated ocean water molecule that get to the space increase the surrounding air temperature as well as the humidity.
 )
)stratification_turbulence. Image credit NASA
While we can say volcanic eruption and ocean current discussed above have in one way or the other contributed to the change in earth environment, the former is destructive while the latter is beneficial for man to be able to live on any part of the globe. However, we human has done more damages while trying to improve on our living standard on the earth through various industrial processing. Some of the important activities in the industry leaves a huge amount of gases hanging in the space. CO2 is one of such gases.

By Alfred T. Palmer - Public Domain,
While the industries are trying to make various process to work they need energy from fossil fuel. While burning fossil fuel gases are inevitably released. The increase in the amount of CO2 in the space is as a result of various industrial activities ranging from combustion to interconversion of one chemical specie to another. These processes has inadvertently lead to the warming of the globe with consequences such as ice melting in the Antarctica region, increase in sea level, and extreme weather . Also Ocean act as carbon storage with about 39,000 GtC (gigatonnes of carbon) in the ocean. This increases the level of acidity in the ocean with consequence decrease in the ocean pH which is too bad for marine biota.
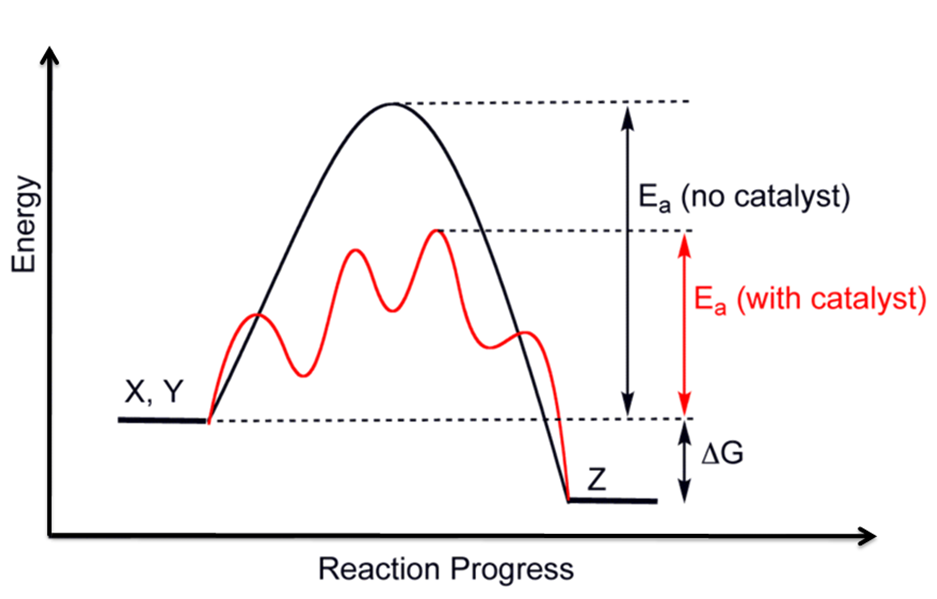
Ever since industrial revolution, scientists have always being working to ensure that there is optimum utilization of raw materials with less production of by-product . Catalyst as being at the fore front in achieving this. Catalyst has some properties which makes them to be useful in the chemical industries. In application of catalyst to industrial processes, it increase reaction rate and helps to get more product of interest .It is known to provide an alternative route to get product of interest with reduced energy for activation just as we see in the red contour under the black curve of the energy profile diagram.
 )
)
Wikimedia Commons, CC0,
Then it is or let say it must be selective for a particular reaction. So we can say that a catalyst is bound to respond to a particular reaction. This last property is what scientists always want to improve most on catalyst. Its selectivity helps scientists to focus it on a particular raw material to have high yield of product with less or minute by-product.
CO2, which is a greenhouse gas with the help of highly selective catalyst can be converted to more useful product.
Up till the recent times, CO2 is been sequestered largely by plant and ocean, while it has found less usage by human that are involved in activities that largely emits the gas. This gas has helped to trapped heat and we have been suffering as a result of our own activities.
Industrial revolution has helped to solve human problem, but it has at the same time put the globe in crises, however, scientists are helping to put a check on the amount of waste generated in the industries. Lets briefly look at various ways by which CO2 that kills climate has become a raw material for production of array of useful products.
Electrochemical CO2 reduction
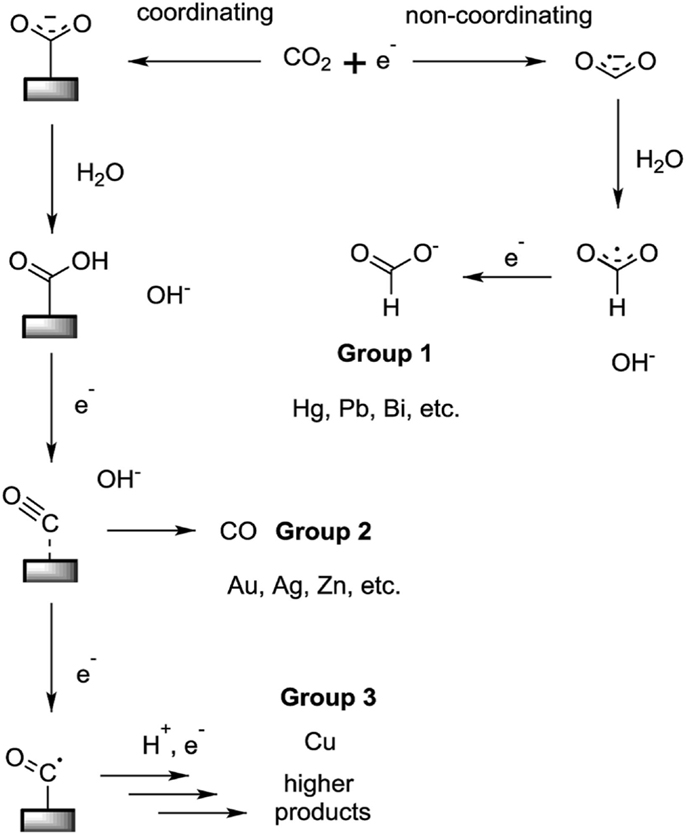
Over the years, many process had been explored to convert CO2 into more useful product. One of such process that helps to convert this climate killer to useful product is the knowledge of electrolysis. This technology is referred to as electrochemical CO2 reduction. In the process, the CO2 is converted to other useful carbon based chemical product. First of all, the gas is made to go through water which is directly placed on the surface of the most important material of the conversion process which is a catalyst. In other to save environment from further increasing the amount CO2, renewable energy source such as solar or wind energy is used to drive the entire process. So what happen is that during the process of passing the CO2 across the surface of the catalyst, one oxygen is extracted from the gas, thereby leaving it with only one oxygen coupled with the carbon (CO) a carbon monoxide. The product now left behind is an important industrial raw materials for the production of many compounds that are industrially relevant and relevant to the end users as well. The most common catalyst for this reaction is Copper.
The above brief discussion can never be possible without an effective catalyst that has all it requires to make the process efficient and produce only a single product. Efficient in the sense that less energy is required to kick start the process and drive it till the end.
The principle of the above process is explained below.
The process of oxidation and reduction come into play here.
Carbon is a group IV element which means that it has 4 electron in it outermost shell. This gives it an opportunity to surround itself with extra four electron being shared with two oxygen atoms through covalent bonding. We can then simply say that Carbon is in it's most oxidized form in CO2,
In other words, carbon has +4 oxidation state in CO2, in making this compound more useful other than it greenhouse effect on the earth, it requires transffering electrons to it so as to reduce the +4 oxidation state. The arrays of reactions that turns CO2 into a reduced state is called CO2 reduction These reactions that leads to reduction Of CO2 can at times be tagged as CO2 hydrogenation when it is thermally induced with H2 , another way in which CO2 is also reduced although this one is the natural process that takes place in the plant Photosynthesis as well as bioinspired catalysis( condition in which the analogue site of an enzyme is built on a electrode material i.e a catalyst behaving like an enzyme for the metabolism of hydrogen.)
Like i earlier put that the CO2 is flushed through water on the surface of Copper the catalyst, the reason being that water will provide the electron and proton required for the reduction, this ensure that no further product is made, this reaction has being demostrated with the first image . Getting result requires modification of the catalyst surface which is one of the ways of improving its performance.
One major thing we need to know about the catalyst is it performance. If the catalyst has low performance, to get the desired product might just be too difficult to achieved. Various ways will be discussed in the subsequent posts on how the performance of the catalyst can be improved to get the specific product.
Summary
Since the time of industrial revolution, the global climate has not really being the same. Man desire to live a quality life has made him to developed technology that require burning of fossil fuel. The result of which lead to the emission of green house gases which then trapped radiation coming to the earth. Thereby increasing the amount of heat on the earth with subsequent effect on the climate. However, in other to reduce the large amount of the CO2 gas being produced, scientists has decided to apply catalyst to convert the
CO2 into more useful carbon based products.
You can join stemsocial on Discord
https://oceanexplorer.noaa.gov/facts/climate.html
https://www.sciencedaily.com/releases/2016/07/160704083006.htm
https://www.catalystseurope.eu/index.php/what-are-catalysts
https://www.sciencedirect.com/science/article/pii/S1878535213003444
The menace that carbon dioxide has created on this planet is little and we humans continue to produce the gas at an alarming rate through various activities. If we can actually start converting the gas to fuel at a large scale, we are on our way to healing the planet. Nice write up
Thanks for coming around.
I guess you wanted to say it the other way round.😁😁
Scientists are working round the clock to ensure that large amount of carbon dioxide is further converted to more useful carbon based products.
I wanted to say 'not little'. An error of omission.
I hope they make a massive breakthrough soon so that we can stop worrying about CO2 and pick on something else to lament on.
We always have what to lament on.😄😄
The gas menace is little compared with the human menace, somehow ;)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support. Using the STEMsocial app could yield even more supporti next time.
CO2 is an issue, for sure, but this is not the only greenhouse gas we should be worried about. Are there searches made to reduce the other classes of gas?