Image made by @yusvelasquez with image public domain
On the other hand, they are almost indispensable in our kitchen, since it is difficult to imagine recipes without some fat; oils and edible fats are also essential for other sectors, especially they are a source of renewable raw material for the cosmetic, pharmaceutical and energy industries.
In this post I want to share with you one of the most used methods in chemical analysis laboratories for the determination of the saponification index, and to establish one of the most important characteristics of oils and fats.
I invite you to read!
That is why the physicochemical characterization of edible oils and fats, as well as the monitoring of changes in their properties that may occur during storage and transport is of utmost importance for different industries, with the purpose of guaranteeing their functionality, quality and the economic value of the products generated from them. One of the chemical parameters used intensively to evaluate their quality is the saponification index, which is a measure used to calculate the average molecular weight of all the fatty acids present in an oil or fat sample[1].
Edible oils and fats are substances consisting of one mole of glycerol and three moles of fatty acids, which are more commonly referred to as triglycerides. A fatty acid is a long-chain monocarboxylic acid, and the length of its chain as well as the number of instaurations determine its physical and chemical properties, i.e., both the number of carbon atoms and double bonds present in its structure. This is extremely important because it is what determines the nature of the fat and serves as the basis for its classification; since fats with longer chains and no unsaturations tend to be solid at room temperature, and we commonly call them shortenings, while shorter chains and those with double bonds are in a liquid state at room temperature, and are classified as oils.
Thus, knowing the type of fatty acid that is mainly present in this type of food is fundamental to be able to estimate its behavior both in the production process and during storage, which is why the saponification index is an important parameter in the characterization of these substances, since knowing their approximate molecular weight will allow us to establish the length of their chain.
Saponification is the base-catalyzed hydrolysis of fats and oils, usually used to produce soap. Triglycerides are esters of glycerol with fatty acids, and when treated with a strong base such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), glycerol and a fatty acid salt (soap) are produced, hence they are said to be saponified.
The chemical reaction involved can be represented in general terms as follows
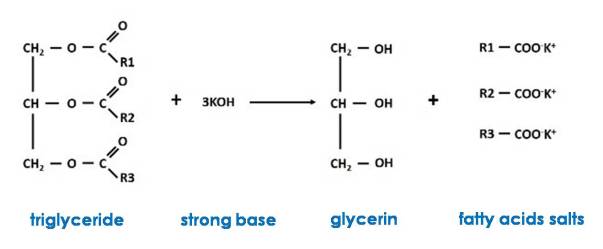
The process occurs in two stages, first the decomposition of the triglycerides occurs and in the next stage the conversion of these occurs to produce three soap molecules. The decomposition of the triglycerides is called hydrolysis, since in contact with water all the esters decompose into glycerin and a fatty acid. But in water only a small part of the fats dissolves in water, so the strong base must be added. During hydrolysis, the OH- ions of the base attack the carbon atom at the end of the molecule where the carboxyl group is located, freeing it from the triglyceride; once free, they react with the sodium or potassium ions to form soap.
Method for the determination of the saponification index.
Principle of the method: This method is mainly based on completely saponifying the sample by the addition of an alcoholic solution of KOH, and the excess is subsequently titrated with HCl.
The saponification index then expresses the number of milligrams of KOH required to saponify all the fatty acids, either free or combined, present in one gram of fat. Fats containing fatty acids with short chains consume more KOH for their saponification than those with longer chains, and therefore exhibit high values of the saponification index; and fatty acids with a longer chain consume less base and therefore have lower values of this index.
If we take into account that in the previous reaction each mole of fat reacts with three moles of KOH, each mole of fat would consume 168 g of KOH, and the saponification index is equivalent to 168000 mg of KOH/molecular weight of the fat. So we can see that the saponification index is inversely proportional to the molecular weight of the fat, and that if we divide by 3 the molecular weight obtained for the fat we obtain the average molecular weight of the fatty acids present in the sample[2].
Method of analysis used
This is a method of analysis adapted from Venezuelan Standard COVENIN 323:1998. Vegetable oils and fats: determination of the saponification index[3].
Materials to be used
- Balance
- Heating plate
- Burette
- Pipette
- Distillation balance
- Condenser
Reagents
- 0.5N hydrochloric acid HCl
- Alcoholic solution of KOH
- Phenolphthalein indicator
Sample
The sample to be used should be transparent, approximately 2 to 3 grams.
Experimental procedure
- Once the oil sample has been weighed, add 25 mL of the alcoholic solution.
- Set up the equipment as shown in the figure, connecting a condenser.

Figure 2. Sample Saponification Source: @yusvelasquez
- Heat in the heating plate until boiling, and verify that the sample is completely homogeneous.
- Subsequently, disconnect the condenser and add the indicator. Approximately 1mL.
- Titrate the hot solution until the color changes from pink to colorless.

Figure 3.Titration of the sample. Source: @yusvelasquez
- Perform the blank test under the same conditions as the oil sample.
Calculations
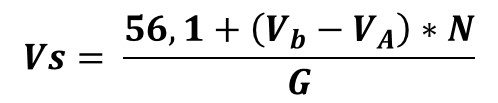
Where:
Vs = Calculated saponification value.
VB= Volume of HCl spent in blank assay titration (mL)
VA= Volume of HCl solution spent in titration of oil sample (mL)
N= Normality of HCl (mol/L)
G= Sample weight (g)
Conclusion and contribution
The saponification index is added to the most important control parameters for the quality control of edible oils and fats, since this index allows us to know the average molecular weight of the fatty acids present in a sample, something fundamental to establish their chemical behavior under different conditions of the productive process as well as of storage and consumption, since this allows to establish the classification and other properties of the fats.
So far this post, I hope that the information presented will be very useful, reminding you that a reliable result in chemical analysis depends on applying the appropriate analytical technique and the appropriate method.
Thanks for reading!
References
- Wikipedia.com. Saponification index].
- Rodriguez, J.A., Maldonado, J.M., Muro, M.A., Miranda, G.A. (2016). Saponification index of five shortenings determined using a micrometer. Research and Development in Food Science and Technology, Vol. 1, No. 1, pp. 937-942.
- COVENIN 323:1998. Vegetable oils and fats: determination of the saponification index.
This is quite educative. How does the saponification index vary with quality? Does a higher saponification index mean higher quality oil and vice versa? Thanks for your contributions to the community.
Greetings friend. Yes, this parameter is related to the quality of the oil, a high saponification index indicates a high purity of vegetable oil, there are standards that establish a minimum value of this parameter for edible oils, since low values of this index indicate a greater presence of long chain fatty acids, which are more related to increased obesity and cardiovascular disease because they are more difficult to digest and adsorb, and therefore the short and medium chain are preferred for dietary purposes. Thank you for your comment!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Excellent write up.
thank you!