Hello!people,Top of the day to you🥳. We'd be learning something new today,I hope you enjoy it😍.
So,today, we'd talk about fluids.
Fun✔️ right?.
Let's go 🚴🚀
To a layman,if the word 'fluid' is mentioned,they think of things like water,juice and other liquids but as an engineer you are made to know that fluids entail two states of matter,which are "liquids" and "gases".
These states of matter have something in common that makes us classify them as fluids.
Want to find out 🧐?,keep reading.
But first🤗;
WHAT IS A FLUID 🤔
A fluid is a material that deforms continuously under the application of shearing forces no matter how small they may be.
Simply put,a fluid is a material that changes physical form when force is applied to it and would not return back to it's original form as long as the force is being applied,no matter how small the force.
Let's make this practical;
Imagine 🤔 you pour a liquid, on the ground let's say water and then someone comes and applies pressure on the surface of the liquid with the feet,what do you notice 👀;
I know what you are thinking;you get wet feet right 😭🤣,yh,sure you would,but there's more to just wet feet. Let's find out what? 🚀
1.You notice that the liquid doesn't remain in the same form it was when it was still in it's original position (deformation)
2.You notice that as long as pressure is applied on that puddle of liquid on the floor,the liquid still keeps changing form over and over again(continuous deformation).
Cool?right
I'm sure you have a clearer understanding now.
Let's move on.
Now this continuous deformity experienced by liquids and gases due to applied pressure is what makes us classify them as fluids.
Now we know why liquids and gases are called fluids 🎉🥳.
So,if we're to give examples with a tree diagram,we can basically have something like this 👇
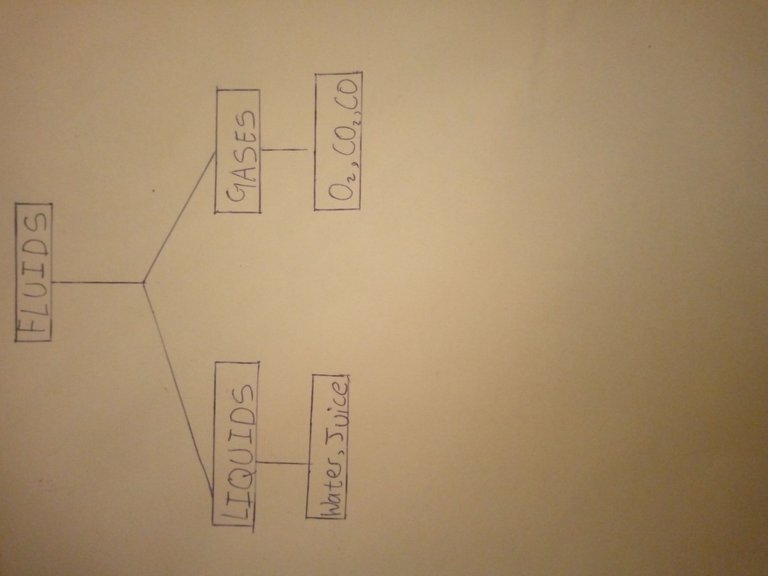
Now there's more to fluid classification than just liquids and gases..hmm.. going from simple to complex right 😢,no worries 🥳😇,stay tuned; there'd be a separate publication for that but let me let you know that this other classification is based on their levels of viscosity i.e (resistance to flow)and they are classified into Newtonian and Non-Newtonianfluids. No worries,I said we'd talk about that later.
Now, as gases and liquids are children of one parent, meaning they are siblings right 😇 and like we humans that have siblings,they definitely have differences 😡😠 with each other.
Getting interesting right 😍.
Well let's look at some of the differences
- Compressibility: Compressibility,which is a measure of the change in volume due to applied pressure is a major problem for liquids,why?well,because the bulk's moduli of a liquid is greater than it's compressibility, liquids are therefore termed as practically incompressible. I see they don't quite agree on that one 😠😡 (stay tuned for more info on compressibility and bulk moduli of fluids in subsequent publications)
2.Volume:Liquids have a definite or fixed volume,what do we mean? if you pour a liquid into a container,whatever volume or space it occupies in the container remains permanent and doesn't change unless you apply pressure
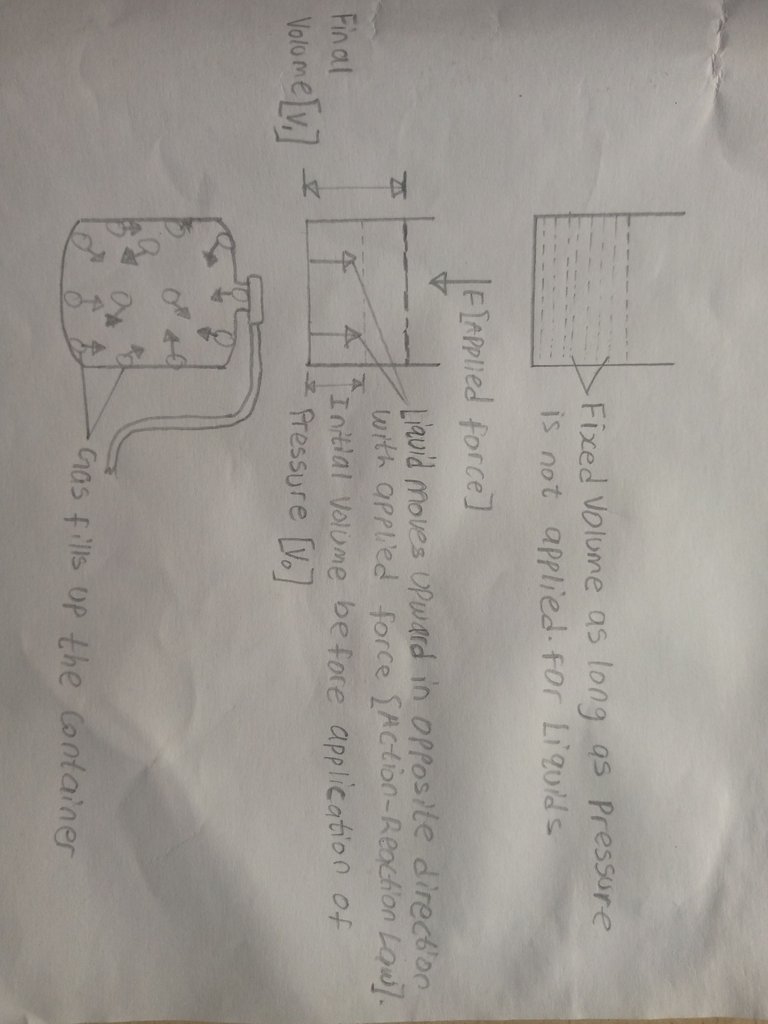
or add more liquid yourself but for gases,they do not have a specific or fixed volume,they fill up the empty space till the whole container is occupied with the gas.
3.Molecular spacing/arrangement:The molecules of gases are well and spaciously separated from each other than liquids
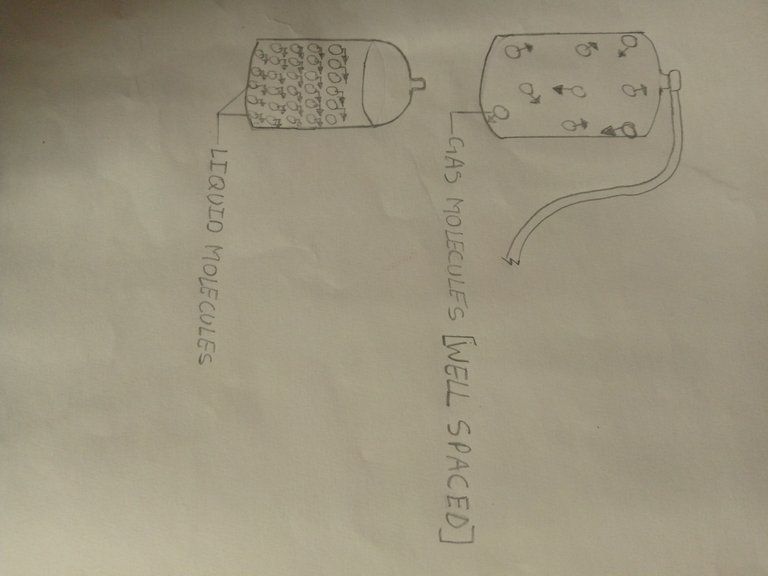
this is the reason they heat up and cool faster than liquids and solids. You can discover from this that the bane of rate of cooling or heating depends on their molecular spacing,which gases are on top of.
Yay! We'd stop here today,Dear reader,I hope I was able to share knowledge successfully and that you found this interesting🙈. please leave comments if you found it interesting.
See you later and have a great day ahead 🚀🚀