For more than 200 years or since the beginning of the industrial revolution, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to the burning of fossil fuels and the change in land use (for example, the increase in car emissions). and deforestation). Meanwhile, the pH of the ocean's surface water has decreased by 0.1 pH unit. The pH scale, like the Richter scale, is logarithmic, so this change represents an approximately 30% increase in acidity.
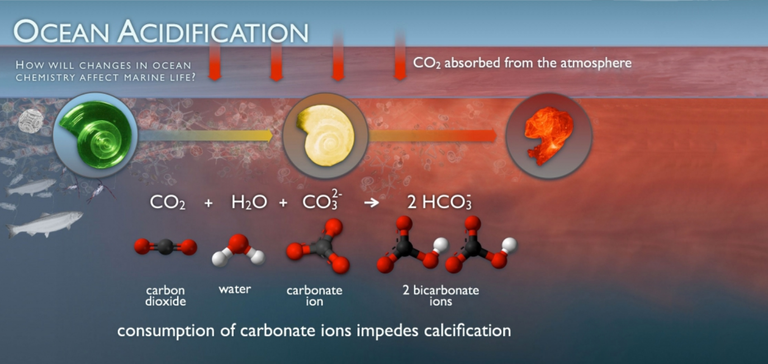
The ocean absorbs about 30% of the CO2 released into the atmosphere. As CO2 levels in the atmosphere increase, levels in the ocean also increase. When CO2 absorbs CO2, there is a series of chemical reactions that lead to an increase in hydrogen ion concentration. This increase makes the seawater more acidic and the carbonate ions are relatively less abundant.
Carbonate ions are an important element of structures such as shells and coral skeletons. The reduction of carbonate ions can hamper the construction and maintenance of shells and other calcium carbonate structures for calcifying organisms such as oysters, clams, sea urchins, shallow water corals, deep water corals. and the calcareous plankton. The pterópodo, or "butterfly of the sea", is a small sea creature the size of a pea. The pteropods are consumed by organisms ranging from small krill to whales and are an important source of food for juvenile salmon from the North Pacific. When the pteropod shells were placed in seawater with projected pH and carbonate levels by the year 2100, they dissolved slowly after 45 days. Researchers have already discovered severe levels of dissolution of pteropod shells in the Southern Ocean, which surrounds Antarctica. Pteropods are small organisms, but imagine the impact of their disappearance on the marine ecosystem!
Changes in ocean chemistry can also affect the behavior of non-calcifying organisms. The capacity of some fish, such as the pollock link, to detect predators is reduced in more acid waters. Recent studies have shown that decreasing pH also affects the ability of clown larvae to link to the site to find suitable habitat. When subjected to lower pH levels, the clownfish larvae lost their chemosensory capacity to distinguish between their preferred habitat and the protective habitat of anemones among the reefs and unfavorable habitats such as mangroves. In addition, greater acidity affects its ability to distinguish between "the smell" of its own species and that of predators. These two factors create a greater risk of predation. When these organisms are at risk, the entire food chain can also be at risk. It is expected that ocean acidification affects many oceanic species. Ocean acidification will harm some species, but photosynthetic algae and seagrass can take advantage of the higher CO2 conditions in the ocean because they need CO2 to live like plants on earth.
Estimates of future levels of carbon dioxide, based on typical emission scenarios, indicate that by the end of the century ocean surface waters could be nearly 150% more acidic, which would generate a higher pH. equivalent to that of the oceans. They have not known for longer. 20 million years.
Ocean acidification is currently affecting the world's oceans, including estuaries and coastal waterways. Today, more than a billion people around the world depend on marine food, their main source of off-site protein binding. About 20% of the world's population consumes at least 1/5 of its animal protein. In the United States and around the world, many jobs and economies depend on the fish and seafood that live in the ocean.
Over the past decade, the ocean science community has paid great attention to studying the potential impacts of ocean acidification. NOAA's Ocean Acidification Program is used to build relationships between scientists, resource managers, policy makers and the public to examine and monitor the effects of evolving ocean chemistry on economically important ecosystems. and ecological, such as fisheries. and coral reefs.
As efforts to monitor ocean acidification around the world are just beginning, it is currently impossible to accurately predict the consequences of ocean acidification on the entire marine food chain and to affect the overall structure of the ocean. 'ocean. Marine Ecosystems As ocean acidification accelerates, scientists, resource managers and policymakerss recognize the urgent need to strengthen science to make sound decisions and actions.