Ballon Fermentation Lab


A fun and highly illustrative lab on fermentation involves Baker's Yeast, sugar, two bottles and balloons. The lab is designed to answer the exploratory question ... Is sugar required for fermentation. First you will have to get your young learner to write a hypothesis.
From our studies ...
Since fermentation requires glucose and releases carbon dioxide, fermentation will occur in bottle A, and the balloon on bottle A will inflate; the balloon on bottle B will not.
Materials
2 bottles (labelled A and B)
2 balloons
Baking yeast
Sugar
a teaspoon measuring spoon


Procedure
- Fill each bottle 3/4 full with warm water.
- Add 1/2 teaspoon of yeast to each bottle .
- Add 1 teaspoon of sugar to bottle A.(Do not add sugar to bottle B) 4. Place a ballon securely on the mouths of both bottles.
- Swirl the bottles to mix the contents.
- Observe and record your observation at 5 minutes
- Observe and record your observations at 15 minutes
- Observe and record your observations at 30 minutes.
- Observe and record your observations at 1 hour.
Prediction
If we put balloons on the mouths of both bottles, we should see Bottle A’s balloon inflate from the carbon dioxide produced during fermentation of the sugar by the yeast. Since Bottle B contained no sugar, fermentation cannot occur and Bottle B’s ballon will not inflate.

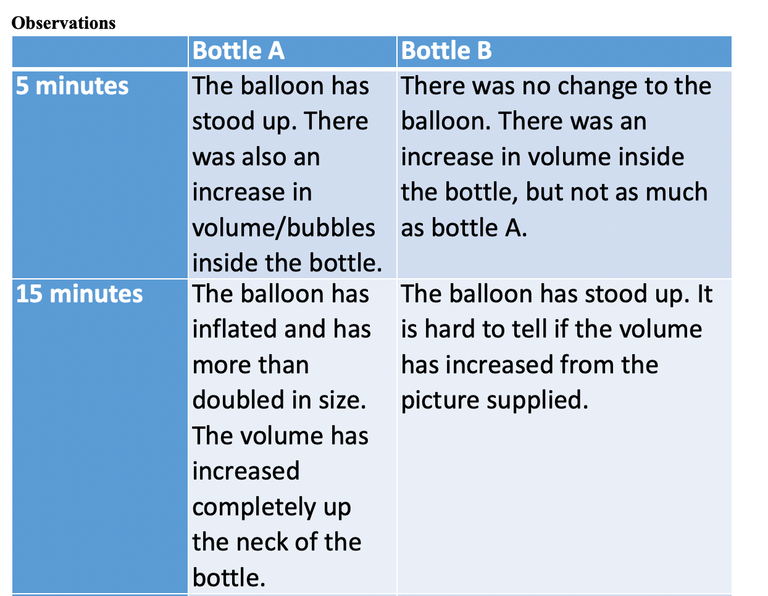


Analysis
Bottle A clearly had fermentation occurring in it. At 5 minutes, the balloon had stood up and there was an increase in bubbles/volume in the bottle, suggesting the production of carbon dioxide due to alcohol fermentation. The inflation increased all the way up to I hour. The volume/bubbles increased until they entered into the balloon and could no longer be observed.
Interestingly, by 15 minutes the balloon on bottle B had stood up and the volume/bubbles in the bottle had also increased, but by a lot less then bottle A. By 30 minutes, the ballon had also inflated a small amount on bottle B. At I hour, both the inflation and the volume/bubbles had continue to increase by a small amount. Some chemical reaction had also occurred in bottle B, perhaps also fermentation.


Bottle A received one teaspoon of sugar. But B did not. Fermentation requires glucose. The sugar in table sugar is sucrose. Sucrose is made up of the monosaccharides, glucose and fructose. The table sugar would have supplied the fuel for fermentation by the yeast. However, observations suggest that a small amount of fermentation also occurred in Bottle B. We will discuss possible reasons for this observation in the conclusion.
Since alcohol fermentation also produces carbon dioxide, carbon dioxide gas is likely contained in bottle A’s balloon. Gas rises and the balloon would not allow its escape to the
Source: Dowse, P. (2021, October 25). What is table sugar?. Sugar Nutrition Resource Centre. https://sugarnutritionresource.org/news-articles/what-is-table-sugar

Conclusion
It would appear that sugar/glucose is required for a high level of fermentation by yeast. Greater fermentation occurred in bottle A. However some chemical reaction, most likely also occurred in bottle B. I believe it to be fermentation. Possible reasons for this include the following .The bottles were not washed thoroughly and still contained small amounts of glucose. The other possible reason, the yeast cannibalized itself to obtain fuel for its cellular function.

If I were to redesign this study, I would use taller bottles so I could observe the increase in volume/bubbles longer. I would also make sure the bottles were very well cleaned and rinsed with alcohol. To study whether or not yeast cannibalization is occurring, I would add a third bottle with an increased amount of yeast. If fermentation is occurring due to cannibalization, I should see greater inflation of the balloon and a greater increase of volume/bubbles then bottle B.










Very well thought out! Next time though, use plastic bottles. Add freshly grated ginger to bottle A. Squeeze bottle A to remove some of the air. Once bottle A regains its shape and feels firm, refrigerate, pour, and enjoy homemade gingerale.
Haha ... this is a good comment.
Or, add this and make your own cola. Or, for the very ambitious, hops, grains and brewers yeast :) Cheers!

Hops ... now you are talking:)
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
The experiment outlined in your post is an interesting one. I find it interesting even though I did little of science in highschool, maybe I'll even replicate the experiment myself I think that will be a fun experience. Thanks for writing and have a nice day.
Earlier I didn't know much about this flower after reading your post and seeing the ran pictures my knowledge has increased a lot it is a very beautiful flower.