Today, I worked on a handout about organic chemistry for first semester students. This did not exactly make me learn too much new information, but I want to share a very fundamental concept of chemistry with you - we are going to talk about chirality. Let's get started with "What I Learned Today #2"!

Molecules and their Mirror Images
Objects, like your hands for example, can be kind of the same, but not really. They are mirror images of one another. If you try to place your hand on top of one another, you will find out that it is impossible to align both your thumbs and index fingers while both of your palms show towards the floor. The same goes for molecules. Some molecules have the same number of atoms, the same connections between those atoms, even the same appearance as a substance in a bottle. However, they are, like your hands, not the same. They are also mirror images of one another.
Thinking that this has a negligible effect on their effect as a scent for example could not be further off. As an example we can compare the two molecules giving the scent of spearmint and caraway seeds:
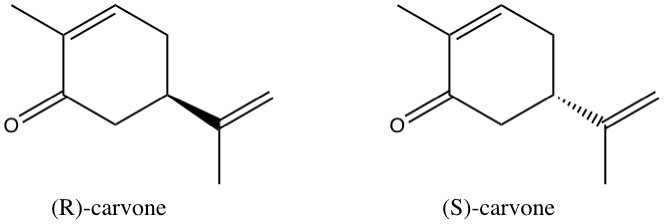
On the left, we have (R)-carvone, which gives the scent to spearmint. On the right, we have (S)-carvone, which looks almost the same, but gives the smell to caraway seeds. The only difference between those two molecules is the full/dashed wedge. On the left side, this means, that the three carbon atoms attached to the ring through the wedge are in front of the plane of the ring, whereas on the right side, they are behind the plane of the ring. If you had a molecule modular in front of you and actually built models of the two molecules, you would see that they are mirror images of one another. It is impossible to align all bonds of the two molecules, just as it was impossible to align your fingers before.
As you can see, those small changes in a molecule can have huge effects on their characteristics. There is a number of examples of drugs which can be either ineffective or very dangerous if you use the wrong form. The problem is, that usually both forms are identical in their physical properties and synthesising only one of the two makes the job for a chemist lot harder. Luckily, scientists around the world are working on new ways to synthesise molecules this way continuously!
Thank you for reading and if you have any questions about chemistry, I will try to write a post about it! Just ask them!
You have not had enough science today but are tired of doing it yourself? - Try out Gridcoin and earn some money!
Gridcoin is a cryptocurrency based on the BOINC network. Instead of producing hot air by calculating meaningless hashes, you work on real scientific workloads with your computer and get rewarded for it. You don't have the newest GPU-farm at home? No problem! You can crunch with your CPU or your GPU and make a decent amount of GRC even with older hardware. Further information can be found here or here and there is even a pool that is pretty much free of charge here.
Great reminder in case you forgot this from elementary school chemistry classes.