
Stained glass, like you see in medieval churches for example, sometimes looks a little odd. At the bottom, they are often thicker than at the top. Many people, tour guides mostly, say that this is evidence that glass is a liquid. How could it move from the top to the bottom otherwise?
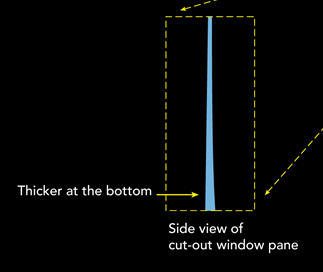
Off course, these people understand that a liquid is not a hard material, so they conclude that glass is a supercooled liquid.
In fact, glass is neither a solid or a liquid, it’s an amorphous solid. An amorphous solid is in a state somewhere in between solid and liquid. I know, pretty vague, but I will give a shot at explaining it.
Solid materials have a very organized molecular structure. They have a crystalized shape, like sugar and salt. Glass however, doesn’t have that crystalized order. Glass has a more organized order than liquids, and molecules can hardly move around, but they also don’t have the structure like a solid. That’s why it is in between solid and liquid.
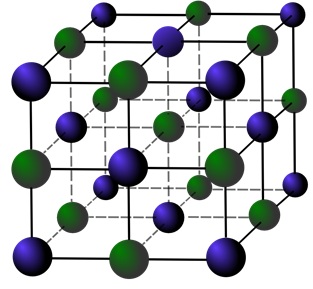
A crystalized structure of a solid. The molecules can’t move around, and have a certain order that is repeated. This forms a solid.
When glass is made, the liquid material is quickly cooled from its liquid state, but is doesn’t solidify when its temperature goes below its melting point, as we normally would expect. If that happens, we speak of a supercooled liquid, also an intermediate state between liquid and solid, but to my understanding, in this state, molecules can still move around a little better than in the amorphous state. After this, the material is cooled down further, below the glass-transition temperature (the temperature at which the material becomes actual glass). At this point, (almost all) molecules can’t move around anymore, making it an amorphous liquid.

The molecular structure of glass in 2D. It doesn’t have a repeated order, like a crystalized structure of a solid.
Over time, the molecules move around a tiny bit, but a mathematical model shows it would take longer than the universe has existed to rearrange molecules from the top to the bottom at room temperature, as seen in churches. So, this does not explain why the glass is thicker at the bottom.
The answer however is quite simple, it probably depends on how the glass was made. At that time, glassblowers made glass cylinders, which they flattened to make glass panes. This technique however was probably not perfect, so some places were thicker than others. Workers who installed the panes probably found it easier to work to keep the thick sides at the bottom. That’s why it gives them a molten, liquid look, but they are an amorphous solid.
If you liked this article, please upvote, resteem and follow. Thanks!
Sources:
https://www.oxfordsparks.ox.ac.uk/crystal
https://www.scientificamerican.com/article/fact-fiction-glass-liquid/
http://www.explainthatstuff.com/glass.html
http://www.huffingtonpost.com/2014/05/03/stained-glass-windows-photos_n_5256052.html
Love it, I have done a similar post on this too :)
Please check out my challenge to maybe participate, and be a part of the biggest Science community on Steemit!
Thanks! I will check it out!