Death in the Morning
It was Friday, the first of December at a local church in South Eastern Nigeria. The pastor needed only one reason to ask his congregation to converge but he had two. It was Friday and it was the first day of the month. He found it necessary that his flock gathered by 9 pm for an all-night praise and worship session, committing his congregation's activities for the month unto God's hand.
This post in not about the pastor or his congregation but they are somewhat involved in the story that follows. How could they not? The next morning, fifteen people were found dead in the church. The pastor was not there. The incident was sour and sad and nobody had a clue as to how these church members wound up dead. The police was as clueless as everyone. Gradually rumour arose that the pastor was responsible for the deaths in his church. The police questioned him and found out that these members were alive and well when the pastor left for his home.
At 4:00 am when the night virgil program ended and the pastor and most of the church members went home all the members who left the church at the same time were in good health. The rest who waited behind and slept in the church until the breaking of the day, we're found dead by 7:25 am when cleaners came to clean the church. So there were no witnesses to account for what happened in between.
After pondering this matter, the District Police Chief realised only this: the victims were people who had no private means of transportation, therefore they had to wait behind until public transport was available when the town woke. But this brought him nowhere close to figuring out what had happened to these men and women. There was only one piece of evidence to rely on: the bodies.
Without any coroner's office in place, he called his friend, Charles, the Medical Director at the Federal Medical Centre to help his conduct a post mortem on the bodies. Charles sent an emergency team to bring the bodies to the morgue. Preliminary examination revealed that there were no physical injuries on the bodies.
Later that evening, Dr. Charles Onima called the police chief. "Hello, Charles," the chief answered. "What do you have for me?"
"It appears, they were all killed by gas poisoning, chief," he replied.
"By whom? How can that be?" the chief queried.
The Silent Killer
Hydrocarbons are organic compounds formed completely by covalent bonds between carbon and hydrogen. This is possible because carbon has four electrons orbiting it's outermost shell such that to attain stability, it needs to form a covalent bond with each of these electrons and it does this easily with hydrogen to form hydrocarbons.
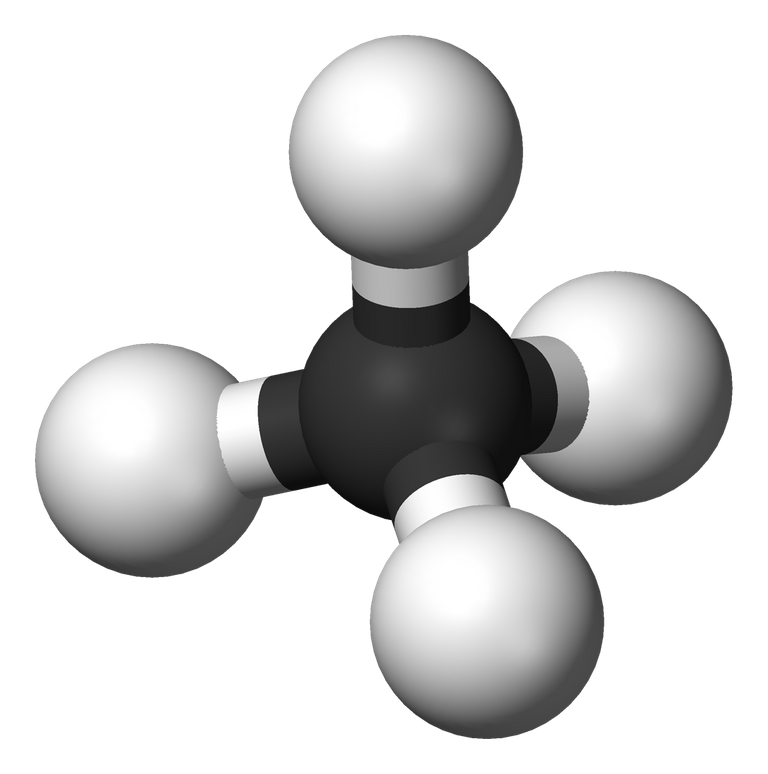
Wikipedia image:
Showing the single bond between four hydrogen atoms and a carbon atom in a hydrocarbon (methane) which is one of the alkanes in the homologous series
Most hydrocarbons occur naturally in crude oil which is refined to produce gasoline, diesel, kerosene, among others. Most of these components are burned daily in engines and machines to generate energy. When hydrocarbons burn in excess oxygen, water and carbon dioxide (otherwise called Carbon II Oxide, CO2) are produced.
For instance when methane burns in oxygen:
CH4 + 2O2 = CO2 + 2H2O
This is an exothermic reaction so heat is also produced. That's the whole point afterall. Naturally, CO2 is released into the atmosphere where it goes on to cause problems of its own which are felt in the long run. Story for another day.
However, when hydrocarbons burn under a limited supply of oxygen, O2, a different compound emerges, carbon monoxide, CO. Of course mono implies that there is only one oxygen atom in this compound.
CH4 + O2 = CO + H2O (unbalanced)
Carbon monoxide is a colourless, odourless, tasteless gas, therefore its presence is not known, making it a dangerous substance. It is produced in combustion engines of cars, electric generators powered by gasoline and diesel. It can also be produced by faulty home cookers or anywhere hydrocarbons are burnt under low ventilation or burning cigarette. But the very invisible nature of this gas is half of the problem.

In most countries, the government’s recommended maximum safe CO concentration is 10 ppm (parts per million). Human beings usually do not feel any different after inhaling up to 70 ppm of carbon monoxide. But at concentrations higher than these, they begin to experience symptoms like fatigue, nausea and headaches all of which are symptoms of flu thereby persuading victims to write carbon monoxide poisoning as flu. At higher levels of up to 160 ppm or more, carbon monoxide poisoning could lead to death.
How Does Carbon Monoxide Kill?
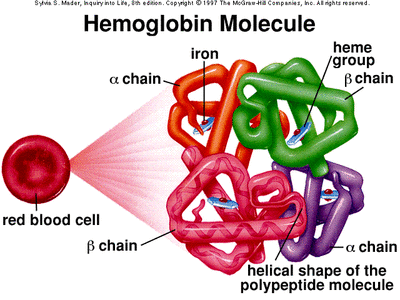
The oxygen that enters the body through respiration, enters through the lungs and is picked up by red blood cells. This oxygen is needed by the cells in the body which efforts make and keep you the way you are. So the red blood cells transport this precious oxygen to your cells.
The main oxygen-carrying substance in red blood cells is the protein haemoglobin. Each haemoglobin molecule contains four iron-containing units called haem groups. Each haem can pick up one molecule of oxygen (O2) each haemoglobin
molecule can carry up to four oxygen molecules. It carries this oxygenated blood through the haemoglobin (now oxyhaemoglobin) and releases some oxygen at cells that are low on oxygen.
The equation below captures this process.
Hb(aq) + 4O2(g) = Hb(O2)4(aq)
- Hb(O2)4 represents
oxyhaemoglobin - (g) and (aq) represent the physical property of either being a gas or aqueous.
The reaction is both forward and backward reaction. The forward reaction takes place in the lungs, the back reaction at cells around the body that are low in oxygen.
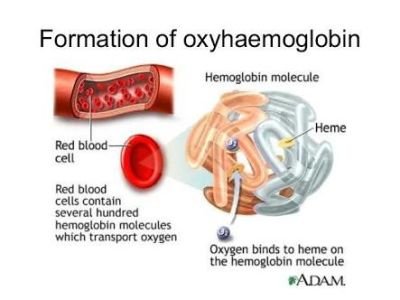
Carbon monoxide kills because it interferes with this process. Because it is more than 150 times better at binding with haemoglobin than oxygen is, inhaling it would cause haemoglobin to bind with it instead of oxygen forming a compound called carboxyhaemoglobin:
Hb(aq) + CO(g) → HbCO(aq)
Since the carbon monoxide sticks so tightly, there are fewer places for oxygen to bind to
haemoglobin when carbon monoxide is present. But carbon monoxide does something else as
well. It changes the other three oxygen binding sites on the haemoglobin molecule so that they
can pick up oxygen but do not release it when they get to the body cells. This means that the
haemoglobin stops acting as the body’s oxygen delivery system.
The problem is even worse for unborn babies. Their haemoglobin is different from that in older
people and is even better at binding to carbon monoxide. This means that unborn babies
occasionally die from CO poisoning, even when the dose is not lethal for their mothers.
Treatment
In less severe cases, bringing a person who has been poisoned with carbon monoxide where they can breath fresh air would reverse the problem in less than six hours. In severe cases, the person might need to be treated in the hospital where oxygen may be administered until full recovery.
Mystery Solved...
At the end of prayer that morning of 2nd December, the remaining members in the church closed the windows and the doors to keep out the cold harmattan wind of that morning. They slept. They did not know that the fumes from the small electric generator that was in use, filtered in through a crevice in the back wall.
They must have been too fatigued to move by the time any of them realised there was something wrong. They all died of the poison.
I hope this has been informative for you. Thanks for reading.
Unless otherwise stated, images were obtained from pixabay
With <3 and gratitude,
Your boy Kels,
@churchboy
We appreciate the way you have incorporated the story with the lesson. Excellent piece of education.
Thank you for sharing
Thanks a real lot. I thought it would be easier to drive the point home with a little story.
this is very needable post on steemit..thank you very..carry on your activity...
Thanks a lot. Your comments are always encouraging. You're awesome.
thank you my friend..
Today, I learned something new from you. The content is very nice and you explained it very well. Thanks dear @churchboy for sharing with us. I like it and I m waiting for your next post.
Thanks for reading. I'm glad you liked the post. I hope to enjoy reading yours as well
Yahh, I enjoy your every post. you are very good.
We have posted a very important one on the platform for them in your post, I think a lot of useful and useful posts to share with us.
Thanks a lot.
Welcome boos
Awesome! I'm so glad #steemSTEM found my post deserving of an upvote. I am very encouraged. Thank you @justtryme90. You're too kind.
Nice post. I wonder how you manage to write on such a broad range of topics. Thanks for sharing.
Thanks a lot. Your comments are always encouraging. You're awesome.
Actually, you are very right we are going killed myself day by day, that's a great post I like this.
Thanks.
Find out many unknown information by your post. thanks for sharing.
Thanks for stopping by. You're appreciated
Very educative post, perfectly blended with a story. Thanks for sharing
Thanks a lot.
Informative post. Well done!
Thank you.
Very articulate post as always. I learnt one or things. Thank you for posting.
Thanks beautiful @royalrose. Your read your comments is to take courage.
You are welcome.
Very informative. All thanks to u on behalf of the pastor.
i most confess that despite being new here, i have learned a lot from your post and these one is quite informative and tells of the beautiful mind of the writer. @churchboy
I am humbled by your compliments. Carbon monoxide poisoning is a topic that affects me personally because I have witnessed it brutally cut short young lives.
Thank you for visiting my blog. You will like it here. Please, stick with it. Welcome to steemit.