
Ever spot the five-year-old bottle of honey at the back of your kitchen cupboard and wonder if it's time to throw it out? Fear not; harmless crystallisation (the result of supersaturated soluble sugars coming out of solution over time) aside, the honey will likely still be perfectly safe to eat - and immersion in warm water will quickly redissolve the sugars.
But what grants honey, on organic substance, such a long shelf life?
The Chemistry Of Honey
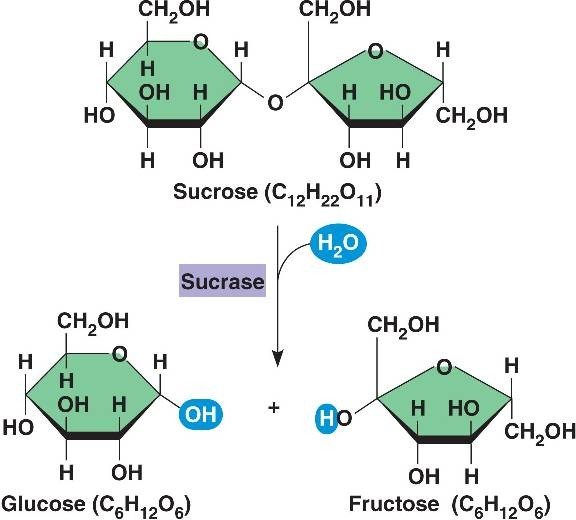
Honey is the product of bees’ conversion of nectar, a watery solution of sugars gathered from plants mainly comprising fructose, glucose and sucrose, into a supersaturated solution of fructose and glucose. Sucrose in the nectar is inverted in a reaction catalysed by the bee-produced enzyme invertase. This inversion of sucrose (a disaccharide comprising two sugars, much like an organic polymer) to glucose and fructose by hydrolysis (addition of water) results in a mixture of monosaccharides which, along with the water in which they are dissolved, constitute over 95% of the honey produced - a high sugar content is responsible for many of honey’s beneficial properties.
Much of the water in the resultant 'crude' mixture is left to evaporate, encouraged by the fanning of wings; the product, honey as we know it, comprises around 80% sugars (mostly post-inversion monosaccharides) and 16-18% water.
Antibacterial
Honey's longevity is derived from its ability to fend off bacteria, the microorganisms primarily responsible for the spoiling of organic material. This antibiotic ability can be attributed to a number of aspects of honey's chemistry:
Hygroscopicity
Supersaturated sugar solutions have a high solute-solvent ratio, and this makes honey very attracted to water. This, coupled with strong hydrogen bonding potential between water and honey's constituent sugars, is responsible for honey's hygroscopic nature: the environment is such that water within bacteria flows from high concentration along concentration gradients to low concentration in the surrounding honey, through the cell membrane.
In effect, the bacteria are 'sucked dry', and are unable to survive without this internal moisture.
Glucose Oxidase
Another enzyme present in bees' digestive systems is glucose oxidase, and it plays a crucial role in inhibiting bacterial growth by catalysing the reaction of glucose with dioxygen to produce gluconic acid and hydrogen peroxide.
Gluconic acid has a pH lower than 4, making it over a thousand times more acidic than the ideal (neutral) environment for bacteria.
Furthermore, hydrogen peroxide is a strong disinfectant and kills bacteria by chemically breaking down their cell walls.
Although glucose oxidase is not active in pure honey - the catalysis requires an abundance of water - its purpose seems to be to effectively sterilise the crude mixture before and during the drying process.
Antibiotic Proteins
Some proteins produced in the very same bee glands used for the manufacture of honey defend bees from bacterial infection. One such protein is bee defensin-1, which scientists believe remains active in the final honey product.
In essence, we (and the honey we eat) are given a short-term 'bee vaccine'!
Methylglyoxal
Certain species of flower produce nectar containing the small organic derivative methylglyoxal, an oxygen-containing organic molecule.
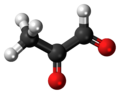
This molecule bestows upon honey its antibacterial ability.
Manuka honey, produced from the nectar of New Zealand's Manuka flower, contains enough methylglyoxal to make it particularly effective in combating bacterial infection, to the extent that it has recently been clinically trialled in New Zealand and North America for the treatment of burns and surface wounds, and is used as a veterinary medicine and home remedy across the world.
Microbial Revenge...
Clostridium botulinum bacteria begin life as harmless non-living spores, which nevertheless prove difficult to kill by means of acidity and hygroscopicity. Upon maturing to bacteria, however, they release the botulinum toxin, which has a fatal dose of 100 nanograms (the weight of one sixth of grain of sugar).
The bad news is that an estimated 10% of honey samples contain clostridium botulinum.
Fortunately, a healthy adult immune system is able to kill the spores before they become toxin-producing bacteria.
An undeveloped immune system, on the other hand, is unable to recognise and combat the foreign bodies, and so it is not recommended honey be given to children under the age of one.

If you enjoyed this article and want more, follow the everyday science blog for your daily dose of science.
References:
Hank Green's Sci Show
A previous article on non-Newtonian properties of honey
www.honeybeesuite.com
Clinical trials of Manuka honey
Nicely presented !!
Methylglyoxal was still controversial as it is also potentially toxic (it is a strongly electrophillic!)
btw, after some search, I found that a grain of sugar corresponding to 0.000000625 kg(which i didn't know before haha), meaning that 100 ng should corresponding to 1/6250 grain of sugar instead.
You're right! Looks like a prefix error. Thanks for spotting it.
I didn't know the reason for not giving honey to kids! Will be more clever tonight ;)
Sometimes there's sense behind the health-and-safety brigade!
More than sometimes I would say ^^
Interesting post
Followed and upvoted.
Kindly do same for me
Check out my latest post
https://steemit.com/steem/@mickyscofield/how-about-a-steem-debit-card
Glad you enjoyed.
There is a lot of fake honey in the stores, make you buy the real stuff.
@cjrc97 Thanks for publishing this Charming instant in time for yourself. Upvoted.
Superior working day I respect all the data and effort thanks :) Upvoted.