At first thought, it seems unremarkable that bodies of water freeze from the top down; after all, why wouldn't they? Surely the surface layer is closest to the cold air and should freeze in place first?
In fact, almost every liquid freezes bottom-to-top, and it's only thanks to a particular quirk of chemistry that water bucks this trend.

Water At 4 °C
In general, a decrease in the temperature of a substance represents a loss in average molecular kinetic energy.
Essentially, colder molecules 'move' more slowly and the volume these molecules occupy is less than that of a hotter, more energetic fluid. It follows that the density (mass per unit volume) of a liquid increases as it cools and its constituent molecules occupy less space.
Water behaves no differently - that is, until it reaches 4 °C (39.2 °F).
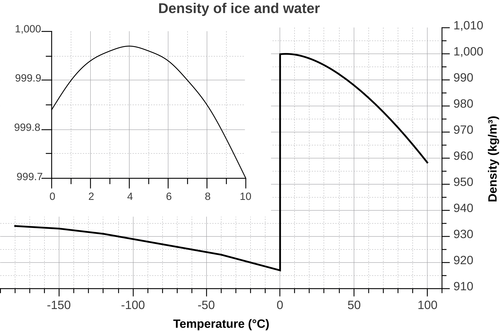
Very cold water is less dense than water at certain greater temperatures, and ice less dense still.
Below 4 °C, the expected trend reverses, and the cooling water becomes less dense with decreasing temperature.
This produces the result that ice is less dense than water at everyday temperatures, which explains why ice cubes float in water and lakes 'freeze over'.
It is also the reason that a can of soda bursts when left in the freezer; the water component of the drink increases in volume, compressing trapped air and pressurising the can beyond breaking point.
Hydrogen Bonding In A Lattice
To explain this unique characteristic, let's consider the chemistry of water nearing the solid-liquid boundary.
Although the exact structure of liquid hydrogen dioxide is unknown, we do know that water at 4 °C comprises water molecules interacting by transient intermolecular hydrogen bonds.
These attractive hydrogen bonds make water relatively dense for a pure (unimolecular) liquid - oil and other simple organic substances tend to float on water.
However, as the water begins to transition from the liquid to the solid phase, a sort of molecular reshuffling occurs.
Previously free molecules experiencing short-lived hydrogen bonding become fixed in a more energetically favourable crystalline lattice structure in which there are 4 groups hydrogen-bonded to each oxygen atom.
This so-called open lattice is tetrahedral in shape and occupies a greater volume per molecule than water.
Ice, Protector Of Life
Returning to our discussion of lakes, we can now comprehensively explain why they freeze top-down.
Our original intuition was that the surface layer must be coldest, and this in itself is not incorrect. This is the reason that the very uppermost layer of water molecules freezes first.
However, in most liquids, this frozen crust would sink as, or even before, it forms - after all, hot fluids rise so cold liquids must conversely sink - and layers of solid would build from the upwards from the bottom of the liquid.
In ice, the lighter crust of ice remains atop the liquid, simply thickening as temperatures fall. This crust insulates the water beneath and prevents large bodies of water freezing even in arctic temperatures.
If ice were to behave as other liquids do, the consequences to life on Earth would be profound.
Annually, midlatitude bodies of water would become uninhabitable.
Shallow bodies of water would freeze solid and arctic oceans would become supercooled by a slurry of ice.
Billions of years of evolution would manifest very differently: fish and other aquatic organisms not specialised for sub-zero, icy conditions - in this dimension, most of them - would not survive.
The majority of marine life, at least as we know it, would not be able to stray far from the equator. Chances are it wouldn't even exist.
This particular version of life on Earth would be unrecognisable.

If you enjoyed this article and would like more, follow the everyday science blog for your daily dose of science.
References:
Klaus-Dieter Keller
Discussion of Tardigrades
www.bbc.co.uk
www.wikipedia.org
www.britannica.com
www.physics.stackexchange.com
Good one
About anomalous expansion of water.
Thank you for posting.
Glad you enjoyed.
Congratulations @cjrc97! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP