Introduction
Psilocybe cubensis mushrooms (shrooms|erowid) contain two compounds of major psychedelic interest: psilocybin (PB) and psilocin (PC). The former is the phosphorylated version of the latter. The addition of this phosphoryl group complicates the extraction process considerably.
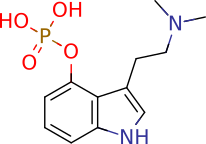
Psilocybin
4-phosphoryloxy-N,N-dimethyltryptamine
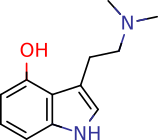
Psilocin
4-hydroxy-N,N-dimethyltryptamine
This post is meant to review the properties of cubensis mushrooms relevant to extraction, the current extraction methods, and to provide a novel extraction method. With the recent decriminalization of certain entheogens, this information should serve as a suitable starting point for manufacturing high quality cubensis extracts on a small to medium scale.
Background
Of utmost importance is the chemical properties of PB and PC. Due to the internal charge interaction between the two OH and one N group, PB is a zwitterion (pKa 1.3, 6.5, 10.4, respectively; calculated). Its water solubility is, therefore, largely independent of pH and cannot be readily extracted by partitioning. In contrast, PC is more amenable to traditional acid-base extraction techniques with a logPOCT = 1.45 and pKa 8.47(N), 11.33(OH).1,2 Since both compounds are present in significant quantities in cubensis, the chosen extraction method must account for these properties.
In addition to the named compounds, P. cubensis also produces a diverse set of β-carbolines and other indole derivatives with potent MAOI activity and mild psychedelic effects of their own.3 While these compounds do have some usefulness in psychedelia, they contribute to many of the negative side effects associated with cubensis (e.g., headaches) and potentially dangerous drug-drug interactions.4 They also prolong and enhance the experience somewhat by inhibiting in-vivo enzymatic detoxification.3 This can be an issue for some consumers, especially with the risk of a "bad trip" lasting longer than expected. Thus, β-carbolines and similar are to be avoided.
Non-psychoactive compounds are also an issue. Cubensis, like all fungi, contain significant quantities of chitin as a part of their cell walls. Cubensis are not cooked prior to consumption like most edible and gourmet mushrooms are, and raw chitin can trigger a significant gut immune reactions in sensitive individuals. Even nonsensitive individuals experience uncomfortable gut effects from ingesting raw fungi.5 Stomach problems and/or nausea are some of the most common negative side effects of whole cubensis ingestion.1 Allergic reactions to mushroom products are not limited to chitin, however.6–8 Beyond the potential side effects, these compounds simply dilute the final product. Thus, chitin and other non-psychoactive compounds are to be avoided.
Cubensis extractions are not inert, either. Cubensis mushrooms produce a phosphatase enzyme which can remain quite active after extraction.2 Coextraction of cubensis alkaline phosphatase and stomach motility could be important factors in the pharmacokinetics of cubensis extracts.
Phosphatase activity is both beneficial and harmful depending on the circumstances. PB is dephosphorylated by innate alkaline phosphatase to produce PC. This occurs primarily in the small intestines, liver, and kidneys of humans.2 The removal of the highly-charged phosphoryl group is a necessary step to convert the pharmacologically inactive PB to the active PC.
PC also crosses the blood brain barrier much more easily than PB.1,12–14 Any method that preserves cubensis alkaline phosphatase will likely accelerate the psychedelic onset of the final product (a desireable attribute) but increases the risk of degrading PB/PC during storage.
Unfortunately, PC is quite labile. It is sensitive to oxidation resulting in the characteristic bluing of damaged cubensis and loss of activity.15,16 For this reason, extraction of fresh mushrooms is wasteful and must be preceded by drying.17 These enzymatic or catalytic degradation mechanisms were unknown or uncontrolled in much of the research literature leading to difficulty in comparing results.18
Exposure to light is also detrimental since it can trigger dimer and trimer condensation reactions for both PB and PC.19 Similarly, dried, whole mushrooms should be stored in the dark until used.17 The extent of this problem for large-scale, complex extraction mixtures is unknown, so it is recommended to avoid light exposure and process cubensis material quickly wherever possible just to be safe.
PB and PC are both intracellular compounds, so the fungal cell wall must be disrupted to release them.20 Some amount of pulverization or maceration is necessary and beneficial for a fast extraction.
The quality of the starting material is an important factor to consider as well. PB and PC concentrations depend on the cubensis genetics, growing, harvesting, drying, and storage methods. Drying and storage methods have a particularly large effect on PB and PC concentrations.17 Significant variations exist from batch to batch, flush to flush, mushroom to mushroom, and even from the different parts of a single fruit body.21 Consuming whole fruit bodies can result in wildly variable effects with no practical method of determining a priori what the outcome will be.
As with all illicit markets, misinformation abounds and contributes to the variability. This public perception of unreliability leads to proliferation of "personal" grow operations and a concomitant increase in variability. For reference, one of the best and oldest sources for this information for both gourmet and medicinal mushrooms alike is the Shroomery forum.
However, the effects of various growing techniques and post-harvest processing methods are not the subject of this post. It is only mentioned here to illustrate that even under strictly controlled manufacturing practices, some amount of standardization will always be warranted.17
Another aspect of general importance is proper formulation of the resultant extract. The difference between a high-purity extract incorporated into a delicious edible and a "toss-and-wash" with orange juice is significant. Both the objective composition of the product and the subjective experience of it are important factors to consider, especially when the experience is so dependent on the "set and setting". Similarly, a "delicious" edible will naturally lead to more buccal/sublingual absorption, potentially altering the pharmacokinetics.3 Therefore, the extract must be amenable to these formulations.
Synthetically produced PB is well documented and the source most commonly used in clinical trials.22 Very little high quality research is done today with cubensis mushrooms or extracts thereof. Synthetic methods eliminate the concerns over β-carbolines, enzymes, or other contaminants derived from natural extracts. However, they are not currently viable for anything other than a clinical setting. In the future, hybrid or de-novo synthesis approaches using submerged fermentation of genetically modified yeasts and bacteria may be viable on the large scale.24 Direct submerged fermentation of cubensis mushrooms has been demonstrated, but is unlikely to proceed beyond lab scale.20,25–29 In any case, some markets will always prefer non-synthetic versions even if the compounds are chemically identical, hence the need for more practical extraction methods.30,31
Likewise, the 4-acetyl version is easier to produce synthetically than PB.22 So-called synthetic shrooms are available for sale in illicit markets. 4-AcO-DMT might be a suitable alternative worth investigating due to the supposed conversion to PC in-vivo, but research evidence is lacking at this time.
The primary goal of any extraction method should be to concentrate PB and PC while minimizing β-carbolines, chitin, and other inactive compounds. Secondary considerations are cost (primarily work-hours), process simplicity, and minimizing solvent use (both for cost and environmental reasons). The method must also be easily scalable so as to minimize variation between batches by averaging. The extraction must also account for the enzymatic and oxidative degradation of PB and PC compounds, respectively, during the process.
Prior Methods
To my knowledge, there are no significant methods available to the community for cost effective and reliable extraction/purification of PB and PC.
The traditional acid-base extraction methods4 used for other psychoactive tryptamines will preferentially concentrate β-carboline impurities and remove PB.2,3 If water is used at all below about 60∘C, PB will convert to PC and oxidation will significantly reduce the psychoactivity. Long periods of open-air evaporation typical of amateur extractions will increase degradation, but even immediate vacuum distillation does not eliminate the problem entirely.
Many recipes for cubensis edibles simply grind the dry mushroom material and directly incorporate the powder into confections. While this does make cubensis more palatable, it does not avoid the gut issues of consuming whole, raw mushrooms. These edibles are also bulky since every portion of cubensis powder must be accompanied by at least as much confection.
Neither can ground cubensis be easily incorporated into candies--desirable and profitable value-added products.32 The stability of such ground products (including the ever-popular ground cubensis in capsules) is questionable.17 Extracts are the only way to make high-quality, reliable cubensis edibles with broad appeal and practical shelf-life. Furthermore, while cubensis occupies a legal and public-opinion gray area, discrete products will likely be more enticing to the consumer than the easily-recognizable whole mushroom.
Extraction techniques that rely on hot water (teas) will have considerable variation due the heat inactivation of alkaline phosphatase and extent of the steeping time. Alkaline phosphatase is a relatively heat resistant enzyme. Five hours at 54∘C is required to reduce the activity of bovine milk alkaline phosphatase to 10% of its original. Only 5 minutes is necessary at 64∘C.33 Similar results were seen with alkaline phosphatase from common fruits and vegetables.34 In my own experimentation, tea made by steeping ground cubensis in cold or warm water is many times more potent and fast-acting than that made with boiling water.
Alternatively, the catalytic oxidation of PC could be partially inhibited by the addition of a suitable chelating agent.15 Ascorbic acid in water is an excellent extraction and chelating system for immediate use. I suspect that this fact contributes to the popular "OJ tek" and "lemon tek". This procedure will not affect enzymatic oxidation, however, so its suitability for large scale is minimal except as an adjunct method.
Of significant, historical note is the "Crystals of the Gods" technique from Professor Fanaticus (of PF Tek fame). PF describes a relatively simple repeat hot 95% ethanol extraction, filtration, and concentration procedure. PF claims that the process yields pure, crystallized PB but efforts to replicate the process have largely failed. The cubic crystal structure in the pictures is unlike the needles or plates reported in the literature for PB or PC.26 Furthermore, the quantity of crystalline product that PF indicated seems to suggest a compound present in much higher concentration than PB.
Regardless of the purity, resuspension of the extract material in a measured quantity of ethanol gives a good product with reduced gut problems. For the amateur, this still seems to be a viable method. However, most amateurs resort to a "fan blowing over a warm bowl" to concentrate their extracts. This wastes a large amount of solvent and time, and it can be hazardous.
Efforts to repeat PF's crystal success over the years were marred with intermittent failure. It was commonly suggested that the high temperature extraction procedure resulted in degraded PB and PC and that a low-temp/long-time extraction would be better--a rumor that later had to be corrected. While that may be true for relative extraction efficiency or PC degradation, I suspect that the absence of heat inactivation of alkaline phosphatase is the source of the intermittent failure.
Other attempts have been made to crowdsource extraction protocols with the usual results. Bioscience companies are also developing their methods but obviously these won't methods won't be made available to the public.
As mentioned above, acidified water is a suitable extraction solvent for immediate use.2 Carbonated water can extract ground cubensis in a few minutes with excellent results. Even iced or hot teas of C. sinensis, which is naturally acidic, work well. Acidified methanol has been used in the literature and is one of the more promising methods for future investigation.17
Thorough extraction protocols are available in the patent literature stretching back to 1965.26
The fruit bodies of Stropharia cubensis Earle collected in Mexico are carefully dried at room temperature in a shady place in the air. 24.2 parts by weight of dried fruit bodies are thoroughly ground and extracted once with 300 parts by volume and then three times with 150 parts by volume of methanol, each time at room temperature for 30 minutes. The extracts are combined and evaporated to dryness under reduced pressure. The residue (6 parts by weight) is defatted by rubbing well four times with 125 parts by volume of petroleum ether and three more times with 50 parts by volume of chloroform each time, the chloroform containing 10% of ethanol. The undissolved residue (59 parts by weight) is dissolved in 5 parts by volume of water. From this solution other products are precipitated by slowly adding 50 parts by volume of absolute ethanol so that the active Substance accumulates in the solution. The purification is repeated two or three times in the same manner.
After this somewhat elaborate and excessively wasteful procedure, the extract is purified further by chromatography and halide removal by silver carbonate. The final yield is approximately 0.8% of combined PB and PC based on the dry weight of the starting material: Not exactly worth the effort.
However, patents (and forums for that matter) are notoriously unreliable so these procedures must be taken as such.
Extraction Procedure
A simple, cost-effective procedure for extracting cubensis is achieved using only methanol and isoheptane (2-Methylhexane). Methanol is the main extraction solvent while isoheptane is used to purify. The result is an ~85% reduced weight extract suitable for edibles. Without the extra isoheptane purification step, the methanol extract forms a sticky resin that is particularly foul tasting. No messy A/B extractions using large separatory funnels are necessary.
Briefly: dry, ground cubensis is extracted three times with equal volumes of methanol over 36 hours in total. The combined extracts are treated with sufficient isoheptane to cause a separate layer on top of the methanolic extract to form (i.e., saturated isoheptane in methanol solution). The solution can be filtered immediately or allowed to settle in a freezer overnight and then filtered. The filtrate is then evaporated and purged (preferably under vacuum) to yield a tan solid.
The necessary solvents can be purified from hardware store chemicals suitable for small scale testing. HEET brand gas line antifreeze is a cheap source of methanol. Before use, fractionally distill it and discard the first 5%. Isoheptane along with diethyl ether are common components of engine starter fluid and can be purified by distillation. As always, commercial OTC products frequently change formulas, so check the SDS of your specific product before buying.
Of course any operation beyond the research level should obtain these solvents from a reputable chemical vendor with proper purity and safety documentation.
Some words of warning: This procedure is not recommended for those without the necessary equipment for (preferably vacuum) distillation. Methanol and isoheptane are extremely flammable. Evaporation in open air is exceedingly dangerous. Methanol is also poisonous. Do not follow this procedure without adequate ventilation or if you are not extremely confident in your ability to purge your final product completely.
The water content of the materials used this procedure is a potential source of unpredictability. For reference, my cubensis are dried at 40∘C for at least 6 hours beyond "cracker dry" and stored with both silica gel and calcium chloride desiccants. My methanol and isoheptane are stored with 3A molecular sieves. The coextraction of impurities is highly dependent on the water content and I suspect that the success of attempts to repeat this procedure will depend on that factor.
Since the extraction procedure results in a somewhat unreliable final weight (± 20%), calculating the final dose based on a ratio to the starting weight is preferred. For example, "1 gram of extract equals 6 grams of dry shrooms." I routinely add an extra 5% to the starting weight to account for mechanical and extraction losses. Large batches help mitigate any variation in the final product.
The key to the procedure is the unusual combination of physical properties of methanol, isoheptane, and water. Small amounts of water present in the initial material and solvents negatively affect the extraction. Isoheptane is sufficiently soluble in methanol to drive any water and water soluble compounds out of solution. Neither methanol nor isoheptane can be used in isolation. Methanol also has the added advantage of denaturing proteins, include enzymes like alkaline phosphatase and allergens, rendering them inactive.
In addition, methanol and isoheptane form a very useful azeotrope. Instead of being forced to evaporate all of the isoheptane at its boiling point of 90∘C, all of the isoheptane will be removed by azeotropic distillation at 59∘C. The lower temperature minimizes the development of burnt or otherwise off-putting flavors. I recommend vacuum distillation to further enhance this effect.
This process works well enough for most confections and some candy products, but research is ongoing to achieve greater purity at a reasonable cost.
Example Extraction
120g (4 oz + 5% excess) of dry cubensis is coarse ground and passed through a mesh kitchen strainer.5 A dust mask, gloves, and a hairnet are recommended for your and the consumer's protection. The powder is added to a stainless steel 500ml french press or larger. Sufficient methanol is added to cover the material making sure that air bubbles have been removed. The press is set aside in a dark place to extract. After 1 hour, more methanol is added to replace the methanol that has soaked into the material.
After 12 hours of total soak time, the plunger is depressed to force out as much methanol as possible (just like making coffee). The methanol is filtered through coarse-grade filter paper or a coffee filter to remove errant bits of cubensis. The filtrate is transferred to a 1 quart canning jar with a lid and stored in the freezer.
More methanol is added to the french press to cover the cubensis material with a bit of stirring to ensure even mixing. The press is set aside for another 12 hour soak.
The mixture is compressed as before and refilled again (for a total of three extractions over 36 hours). For the final press, more methanol is extruded by emptying the French press contents into a nylon nut bag and squeezing manually with gloved hands.
When combined, the tan-yellow methanol solution should have a total volume of 400-600ml.
Isoheptane is added slowly to the methanolic extract with mild stirring. About 50ml is typically required. A white precipitate of inactive compounds will form making the solution cloudy. The amount of precipitate will be proportional to the water content of your starting material and solvents. Sufficient isoheptane is added to form a thin layer of isoheptane on the surface indicating a saturated methanol solution. The solution is allowed to fully precipitate in the freezer overnight (i.e., winterizing).
After winterizing, the solution is filtered while cold to remove as much of the precipitate as possible. I recommend placing a cotton ball in the neck of the funnel with a fluted coarse filter over top. The filtrate is evaporated at reduced pressure to a final volume of 20-40ml and then transferred via pipette to an evaporating dish or small beaker.6 A small amount of warm methanol can be used to rinse the evaporating flask. The rest of the solvent can be purged in a desiccator overnight at high vacuum and mild heating.
The resulting extract weighing approximately 16g can be ground to a fine, tan powder and directly incorporated into confections. For candy, better texture is achieved by suspending the extract in about 4 parts (v/w) absolute ethanol and using it like any other alcohol-based flavoring.
In this example, the final extract weight was 16.84g containing approximately 1 gram of pure PB + PC. Using the starting weight of 113.6g, each gram of extract is equivalent to 6.75g of dry cubensis, or 1 gram of dry cubensis is equal to 0.15g extract. Adjust your recipes accordingly.
A note on recovering the methanol and isoheptane: Methanol that is presaturated with isoheptane in not as effective of a solvent as pure methanol. During extraction, I evaporate the extract as described above and then fractionally distill the mixed solvent at a later time. The fraction that boils below 64∘C will separate into an upper layer of methanol-saturated isoheptane and a lower layer of isoheptane-saturated methanol. The upper layer can be used just as isoheptane would be. The lower layer can be recycled using a combination of salting out and more distillation, but it is simpler and probably cheaper to use it for some other purpose (e.g., an organic cleaner for glassware). The fraction that boils above 64.7∘C is pure methanol and can be reused directly for extraction.7
In total, about 500ml of methanol and 50ml of isoheptane are used, of which, about 75% and 95% respectively is recovered depending on your chosen procedure. The primary loss of methanol is the residual solvent adhering to the extracted cubensis matter. This could be reduced using a hydraulic press, but the cost of the additional equipment and labor might not be worth it.
A second purification step can give a cleaner product at the loss of some activity (magnitude unknown). I prefer to use this additional step when making translucent hard candy. The dry extract is dissolved in 80-100 ml of warm absolute ethanol and allowed to cool. Some inactive residue will remain and can be filtered out although I prefer to centrifuge the extract instead. The extract can be winterized overnight to remove a bit more residue. The tincture can be stored at room temperature in a dark place, but I suggest using it within a week of manufacture.
The combined extraction and purification procedure requires about four days (less with a rotavap) to complete using about four man-hours of actual labor. Total cost of the materials (including solvent loss, filters, cleaning material, desiccating agents, electricity, etc.) is $1-2 for four extracted ounces.
Future Research
Cubensis extraction has a lot of room for improvement. Specific methods for large scale extraction and systematic testing thereof are lacking in the literature. A plethora of forum posts and patents exist that are good for inspiration but lack the veracity of dedicated, high-quality research in professional labs. My work can hardly be considered part of the latter, nevertheless, here are some specific areas that I see as potential sources of improvement.
In regard to improving my specific method, adding a pre- or post-extraction washing step should be investigated. A low boiling point, highly nonpolar solvent should remove more inactive material (such as ergosterol).35 Similarly, the isoheptane layer formed in this procedure would likely contain much of the nonpolar compounds. It could be separated before distillation to remove them. Research is ongoing to determine the optimal ratio of water:acid:methanol:polar solvent and proper ordering.
The extraction period could use some tuning as well. Three extractions over 36 hours was taken as an estimate from the literature to achieve near total extraction efficiency.18 This is definitely excessive and perhaps the least desirable aspect of this method. Some combination of warmer extraction temperatures, finer grinding/maceration, or advanced extraction procedures (e.g., sonication) might shorten this period. It might very well be the case that the second and third extractions only give an extra five percent, for example, and thus may not be worth the extra effort and time. Only more elaborate tests with good analytical methods can tell.
Apart from procedural additions, some steps could benefit from substitution. Isoheptane, for instance, is somewhat rare and I used it primarily because I already had it. Any of the other C5-C8 alkanes should be investigated, particularly hexane due to its commercial availability, relative safety, and low boiling point.
Naphtha is more commonly available to the amateur. However, it typically contains higher boiling point compounds that may or may not form an azeotrope with methanol. In this case, adaptations to the evaporation and purge steps would be necessary.
Depending on the quality of your chosen brand, naphtha might also contain various sulfur compounds and cyclic hydrocarbons both of which are to be avoided. If you choose to investigate naphtha, the best source to start with should contain "light hydro-treated petroleum distillates" and little else. Since so little isoheptane is used and it is almost entirely recovered, I suggest sticking with isoheptane even if you have to buy it commercially.
There are also environmental costs to consider in choosing a good substitute. Methanol can be derived from renewable sources (even though most is currently not) but isoheptane definitely will not be. Methanol also degrades quickly in the environment unlike isoheptane. Replacing isoheptane with a more environmentally friendly alternatives such as 2-Methyltetrahydrofuran or D-limonene should be investigated. The latter might appeal to more consumers due to its natural origin.
Completely different extraction methods are possible as well. Supercritical CO2 extraction could probably be adapted to cubensis extraction. Large scale techniques like that might be worth investigating if and when cubensis becomes legal on the scale of cannabis. Especially considering many of these techniques and equipment are common to the cannabis industry already and might be readily adaptable.
However, PB and PC do not benefit from any coextracted components in the same way as cannabis benefits from coextracted terpenes. So more advanced separation methods like chromatography, which are not typically used in the cannabis industry, are worth investigating. Those methods are capable of producing pure crystals of PB and PC for the most refined edible experience.35 The provided, relatively simplistic method might only be the first step in a more elaborate procedure.
Likewise, this procedure is tested on cubensis but should be applicable to other psychoactive fungi that produce PB. It will most likely work with any psychoactive fungi, such as Amanita muscaria, but that has not been tested.
As mentioned earlier, extractions are not done in isolation: Their success is partly determined by the quality of the starting material and methods. A systematic study of production methods and their effect on final PB and PC concentration should be made. Without standardized or at the very least characterized growing methods, the extract's potency will remain variable and unknown.
Conclusion
Herein is provided a relatively easy and cost-effective extraction procedure for cubensis mushrooms. Much of this procedure has been developed by someone with no formal training in chemistry. There are undoubtedly errors in this post and potential advances that would be obvious to someone skilled in the art of pharmacognosy.
I will continue to stumble my way through the development of better extraction techniques. Great advances beneficial to everyone could be made by someone with access to the proper chemical analysis equipment and a single spore syringe of P. cubensis. I look forward to the next few years as cubensis, and psychedelics in general, gain public and legal approval.
P.S. If anyone has an affordable GC/MS setup for sale, I'm in the market...
Bibliography
1. Migliaccio GP, Shieh T-LN, Byrn SR, Hathaway BA, Nichols DE. Comparison of solution conformational preferences for the hallucinogens bufotenin and psilocin using 360-MHz proton NMR spectroscopy. J Med Chem. 1981;24(2):206-209. doi:10.1021/jm00134a016
2. Casale JF. An Aqueous-Organic Extraction Method for the Isolation and Identification of Psilocin from Hallucinogenic Mushrooms. J Forensic Sci. 1985;30(1):10989J. doi:10.1520/JFS10989J
3. Blei F, Dörner S, Fricke J, et al. Simultaneous Production of Psilocybin and a Cocktail of β‐Carboline Monoamine Oxidase Inhibitors in “Magic” Mushrooms. Chem Eur J. 2020;26(3):729-734. doi:10.1002/chem.201904363
4. Herraiz T. β-Carbolines as Neurotoxins. In: Antkiewicz-Michaluk L, Rommelspacher H, eds. Isoquinolines and Beta-Carbolines as Neurotoxins and Neuroprotectants. Springer US; 2012:77-103. doi:10.1007/978-1-4614-1542-8_5
5. Patel S, Goyal A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. International Journal of Biological Macromolecules. 2017;97:331-338. doi:10.1016/j.ijbiomac.2017.01.042
6. O’Neil CE, Hughes JM, Butcher BT, Salvaggio JE, Lehrer SB. Basidiospore Extracts: Evidence for Common Antigenic/Allergenic Determinants. Int Arch Allergy Immunol. 1988;85(2):161-166. doi:10.1159/000234496
7. Helbling A, Horner E, Lehrer SB. Identification of Psilocybe cubensis Spore Allergens by Immunoprinting. Int Arch Allergy Immunol. 1993;100(3):263-267. doi:10.1159/000236422
8. Horner W, Reese G, Lehrer S. Identification of the Allergen Psi c 2 from the Basidiomycete Psilocybe cubensis as a Fungal Cyclophilin. Int Arch Allergy Immunol. 1995;107(1-3):298-300. doi:10.1159/000237007
9. Hasler F, Bourquin D, Brenneisen R, Bär T, Vollenweider F. Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharmaceutica Acta Helvetiae. 1997;72(3):175-184. doi:10.1016/S0031-6865(97)00014-9
10. Horita A, Weber L. Dephosphorylation of psilocybin in the intact mouse. Toxicology and Applied Pharmacology. 1962;4(6):730-737. doi:10.1016/0041-008X(62)90102-3
11. Horita A, Weber L. The enzymic dephosphorylation and oxidation of psilocybin and pscilocin by mammalian tissue homogenates. Biochemical Pharmacology. 1961;7(1):47-54. doi:10.1016/0006-2952(61)90124-1
12. Tylš F, Páleníček T, Horáček J. Psilocybin – Summary of knowledge and new perspectives. European Neuropsychopharmacology. 2014;24(3):342-356. doi:10.1016/j.euroneuro.2013.12.006
13. Dinis-Oliveira RJ. Metabolism of psilocybin and psilocin: clinical and forensic toxicological relevance. Drug Metabolism Reviews. 2017;49(1):84-91. doi:10.1080/03602532.2016.1278228
14. Geiger HA, Wurst MG, Daniels RN. DARK Classics in Chemical Neuroscience: Psilocybin. ACS Chem Neurosci. 2018;9(10):2438-2447. doi:10.1021/acschemneuro.8b00186
15. Levine WG. Formation of Blue Oxidation Product from Psilocybin. Nature. 1967;215(5107):1292-1293. doi:10.1038/2151292a0
16. Lenz C, Wick J, Braga D, et al. Injury‐Triggered Blueing Reactions of Psilocybe “Magic” Mushrooms. Angew Chem Int Ed. 2020;59(4):1450-1454. doi:10.1002/anie.201910175
17. Gotvaldová K, Hájková K, Borovička J, Jurok R, Cihlářová P, Kuchař M. Stability of psilocybin and its four analogs in the biomass of the psychotropic mushroom Psilocybe cubensis. Drug Test Anal. 2021;13(2):439-446. doi:10.1002/dta.2950
18. Gartz J. Extraction and analysis of indole derivatives from fungal biomass. J Basic Microbiol. 1994;34(1):17-22. doi:10.1002/jobm.3620340104
19. Anastos N, Barnett N, Pfeffer F, Lewis S. Investigation into the temporal stability of aqueous standard solutions of psilocin and psilocybin using high performance liquid chromatography. Science & Justice. 2006;46(2):91-96. doi:10.1016/S1355-0306(06)71579-9
20. Gartz J. Biotransformation of tryptamine derivatives in mycelial cultures of Psilocybe. J Basic Microbiol. 1989;29(6):347-352. doi:10.1002/jobm.3620290608
21. Bigwood J, Beug MW. Variation of psilocybin and psilocin levels with repeated flushes (harvests) of mature sporocarps of Psilocybe cubensis (earle) singer. Journal of Ethnopharmacology. 1982;5(3):287-291. doi:10.1016/0378-8741(82)90014-9
22. Nichols DE. Improvements to the Synthesis of Psilocybin and a Facile Method for Preparing the O-Acetyl Prodrug of Psilocin. Synthesis. 1999;1999(06):935-938. doi:10.1055/s-1999-3490
23. Shirota O, Hakamata W, Goda Y. Concise Large-Scale Synthesis of Psilocin and Psilocybin, Principal Hallucinogenic Constituents of “Magic Mushroom”. J Nat Prod. 2003;66(6):885-887. doi:10.1021/np030059u
24. Milne N, Thomsen P, Mølgaard Knudsen N, Rubaszka P, Kristensen M, Borodina I. Metabolic engineering of saccharomyces cerevisiae for the de novo production of psilocybin and related tryptamine derivatives. Metabolic Engineering. 2020;60:25-36. doi:10.1016/j.ymben.2019.12.007
25. Catalfomo P, Tyler VEJ. The Production of Psilocybin in Submerged Culture by Psilocybe cubensis. Lloydia. 1964;27(1):53-63. http://www.fungifun.org/docs/mushrooms/Psilocybe/The.Production.of.Psilocybin.in.Submerged.Culture.by.Psilocybe.cubensis.pdf
26. Heim R, Hofmann A, Brack A, Kobel H, Cailleux R. Obtaining Psilocybin and Psilocin From Fungal Material. 1965;(56143). https://patents.google.com/patent/US3183172A
27. Leung AY, Smith A, Paul A. Production of Psilocybin in Psilocybe baeocystis Saprophytic Culture. Journal of Pharmaceutical Sciences. 1965;54(11):1576-1579. doi:10.1002/jps.2600541104
28. Agurell S, Nilsson JLG, Liaaen-Jensen S, Schwieter U, Paasivirta J. Biosynthesis of Psilocybin. Part II. Incorporation of Labelled Tryptamine Derivatives. Acta Chem Scand. 1968;22:1210-1218. doi:10.3891/acta.chem.scand.22-1210
29. Fricke J, Lenz C, Wick J, Blei F, Hoffmeister D. Production Options for Psilocybin: Making of the Magic. Chem Eur J. 2019;25(4):897-903. doi:10.1002/chem.201802758
30. Moscato EM, Machin JE. Mother natural: Motivations and associations for consuming natural foods. Appetite. 2018;121:18-28. doi:10.1016/j.appet.2017.10.031
31. Rozin P, Fischler C, Shields-Argelès C. European and American perspectives on the meaning of natural. Appetite. 2012;59(2):448-455. doi:10.1016/j.appet.2012.06.001
32. Charlebois S, Somogyi S, Sterling B. Cannabis-infused food and Canadian consumers’ willingness to consider “recreational” cannabis as a food ingredient. Trends in Food Science & Technology. 2018;74:112-118. doi:10.1016/j.tifs.2018.02.009
33. Claeys WL, Ludikhuyze LR, Van Loey AM, Hendrickx ME. Inactivation kinetics of alkaline phosphatase and lactoperoxidase, and denaturation kinetics of β-lactoglobulin in raw milk under isothermal and dynamic temperature conditions. Journal of Dairy Research. 2001;68(1):95-107. doi:10.1017/S002202990000460X
34. Jakób A, Bryjak J, Wójtowicz H, Illeová V, Annus J, Polakovič M. Inactivation kinetics of food enzymes during ohmic heating. Food Chemistry. 2010;123(2):369-376. doi:10.1016/j.foodchem.2010.04.047
35. Stebelska K. Assays for Detection of Fungal Hallucinogens Such as Psilocybin and Psilocin. In: Neuropathology of Drug Addictions and Substance Misuse. Elsevier; 2016:909-926. doi:10.1016/B978-0-12-800212-4.00084-4
PB/PC lack any direct emetic effects due to their minimal 5-HT3 binding unlike 5-substituted indoles like ibogaine.↩
The only reference that suggests dephosphorylation in acidic conditions is Dinis-Oliveira et al., but the cited references do not corroborate the effect.9–11 Unfortunately, this perceived auto-dephosphorylation at low pH has permeated the community (e.g. [lemon tek][lemon]).↩
Pure PB and PC are quite bitter and slightly amine tasting: Not exactly the ideal substrate of "delicious" edibles. Market research should be done to determine the extent of people's aversion to this poor taste and ways to mitigate it.↩
Any procedure or discussion based on AB extraction or salts of psilocybin will end in disappointment.↩
Ultra-fine powder is not recommended as the filtration step becomes exceedingly slow.↩
I like pyrex agar plates, personally. Low temperature vacuum evaporation leads to a better tasting product.↩
If you are unsure of the isoheptane content of your fraction, add a small amount of water. If isoheptane is present, the solution will become cloudy as isoheptane is pushed out by the polar water.↩
Congratulations @freecatalyst! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
Your next target is to reach 50 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out the last post from @hivebuzz: