Hello Dear Steemian, Today i will share you a familiargemstone used by ancient for treatment, i will explain Scientifically how this gems can use for treatment, , , ,
Amethyst
 Source :reswara
Source :reswara
Amethyst is a very popular gemstone, its purple color can make people our eyes fascinating, and this gemstone also has a high value and demand by many collectors. Some people believe this gemstone can provide protection for its users, such as peace, strength, intelligence, and spiritual. Some others believe this gem can attract the love of a woman, but this is only for the believer ;). Before i discuss about amethyst stone, let us know a bit story about Amethyst gemstone in era of Egyptian ancient.
The ancient Egyptian society believed this gemstone can provide protection for Kings tombs, in the middle of century the gemstone used for treatment, intelligence and protect the user from black magic, in the era of ancient roman the gemstone used to prevent drunkenness, by insert this gems into wine glass.
Lets to Solve This Matter In Science Explain
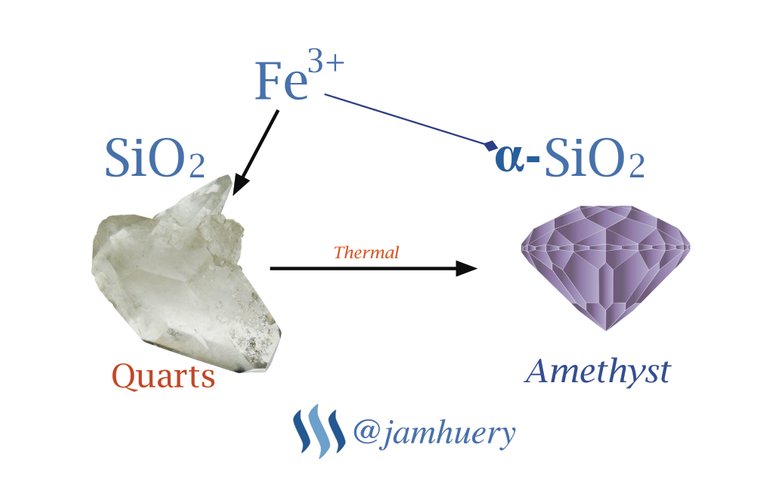
Chemically, amethyst gemstone is quartz mineral compounds composed of Silicon and Oxygen with chemical formula SiO2. The purple or violet color of this gemstone due to chemical impurities, the impurity is Trivalent Iron (Fe3+). The concentration of ion Fe3+ in the gemstones can affect it color, either purple or dark purple, it depend on iron concentration. Because the color affected by impurity, so the chemical formula for Amethyst is ἀ-SiO2, where the ἀ- is impurity, in chemical formula we can write as follow :
Fe3+ + SiO2 => ἀ-SiO2
The ἀ- is trivalent iron which give the color effect for quartz mineral, SiO2 is a Quartz or Silica, when the trivalent iron (Fe3+) bind to the quartz (SiO2 or silica), it change color to the purple, so it called as Amethyst.
What is Quartz ?
 Source :pixabay
Source :pixabay
Quartz is a mineral formed from silicon (Si) and oxygen (O2), it can form at any mineral temperature and it resistant of weathering (corrosion), so the quartz can be found in mountains, beaches, rivers and deserts and it also can be found in metamorphic, and igneous rocks. Quartz is a natural mineral, it has hardness of 7 mohs scale and used for lens manufacture and laser cover.
Chemically, Quartz and Silica are same, it composed of silicon and oxygen, silica can to produce silica gel, it is grain glass with the pores, silica gel can be synthesized with Sodium Silicate, although its name "gel" but it was hard like a glass, i have explain this before how i made it from Bagasse. But I review a bit about it cluster named with siloxane (Si-O-Si), silica gel has a siloxane group, this group in Silica Gell serves as an absorber, silica gel can absorb moisture and also toxic gas. its ability in absorption due to siloxane group, it can survive until heating temperature below 600oC.
Chemistry Answer Scientifically
 Source :wikipedia
Source :wikipedia
Mineral quarts can formed at any mineral temperature, the synthetic of Amethyst gems can be formed at hydrothermal grow or high-pressure autoclave temperatures. Naturally, the forming of Amethyst precious gemstone also occurs at high temperatures and pressures. When Quarts begin to melt and mix with trivalent iron (Fe3+) elements, so, the process changes from quartz to amethyst, When it have been cool, the mineral begins to be harden and occur color change, the basic element of Amethyst forming is silica (quartz). Where the silica also contain the siloxane that serve as absorber, so this was reasonable if the ancients insert the Amethyst gemstone into wine glass to prevent drunkenness, because amethysts with siloxane (cluster) also has function as an absorber.
Conclusion
Conclusion
The purple color of amethyst gemstone caused by chemical impurity, Amethyst gemstones there were formed naturally and synthesis, to synthesize amethyst should to use high temperature.
Source :
Image CC Source
Support Scientist By Use #science tag or join @steemSTEM
Follow Me @jamhuery

Find me one source indicating that amethyst interacts with ethanol or reacts with it, or how that would even make any sense. Because while you gave a nice description of the rock, and why it has it's color, you did not provide any evidence in favor of it's ability to prevent drunkenness.
I this myth is just that, an untrue myth. Amethyst is pretty, and people had weird superstitions in the past. They wore it as a talisman and put it in their cups, but it didn't stop them from getting drunk. It didn't do anything other than look neat. Siloxanes or not.
Very good question, and this open my mind to do some research, i will answer with some sense.
indeed it is based on a mythology, but Perviously, i have made a thesis about silica xerogel (dry),
in the manufacture of silica gel, we must burn a material contains SiO2 inside,
after it heating at high temperatures, silica ash reacted with NaOH to obtain sol sodium silicate, then this sol soaked with acid to forms a gel, then the gel dried and silica xerogel has been forming, determination of silica coincides with siloxane formation that serve as toxic gas absorber. Indeed there is no reaction between amethyst and ethanol, but siloxane react with alcohol can to form silanol.
At room temperature?
With out this, then science can not justify the myth ;)
It also means:
That you can't conclude this. Because the people of yesteryear were not correct, amethyst did not prevent drunkenness.
yes it is, thanks for feedback, it mean my conclusion was wrong, i have to change it now,
Had to take a closer look at your site for this, and it was much there i have to read later.
you have a new follower in me. :)
thanks nice to meet u, i
I like the scientific part here.
I know minerals very well.
I have also made Citrine out of Ametyst many times as a experiment, by warming up Ametyst. :)
When i saw the post first, i must admit that i did not actually expected the one that write it to know the chemical form of it and so.
I am impressed. :)
Thanks for sharing, even if i am not so sure about all the details about how to use it.
But i will not say it is wrong, because i have never researched that part.
I love this crystal! ♡
And me too, but it has hight price, hehe
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by jamhuery from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews/crimsonclad, and netuoso. The goal is to help Steemit grow by supporting Minnows and creating a social network. Please find us in the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
Very interest. I like your post @jamhuery.