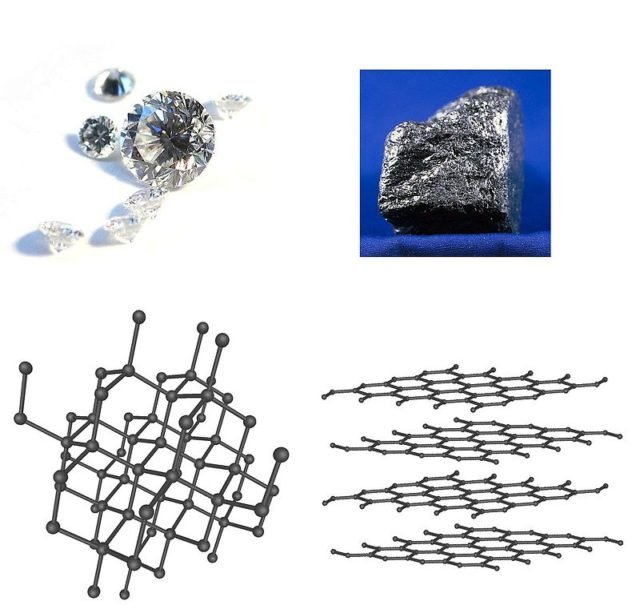
Diamond, graphite and its structures. Source: APS Physics.
Alotropy is the ability of some elements to have different atomic or molecular structures. Each of these forms is called allotrope. Carbon is capable of forming many different allotropes due to its ability to form different types of chemical bonds. The best known forms of carbon include diamond and graphite (the pencils mine). In recent decades, many more allotropes and carbon forms have been discovered and investigated, including spherical forms such as buckminsterfullerene, sheets such as graphene or nanobelts and tubulars such as nanotubes. It is estimated that there are around 500 carbon allotropes.
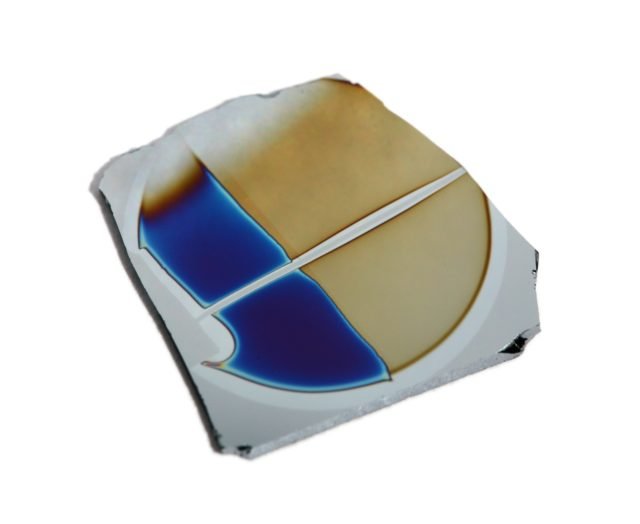
Diamond-like carbon (DLC) sheet on silicon. Source: APS Physics.
Amorphous carbon is the allotrope of carbon that does not have a crystalline structure. As with all vitreous materials (so-called glass), some short-range order may occur, but there are no long-range patterns of atomic positions. A variety of amorphous carbon is the so-called diamond-like carbon, more commonly known in the industry by its acronym DLC (diamond-like carbon)
Chemists and engineers have learned by trial and error to create DLC films by depositing carbon atoms on a carbon substrate. The use of trial and error is precisely because it is not theoretically understood how these films are formed. Now, by combining an automatic learning algorithm with molecular dynamics simulations, Caro and his colleagues have shown that DLC films are formed through a "shot peening" mechanism.
If in the blasting of a sheet metal workshop, the body of a car is treated with a "shot of grit" (or "sand") to leave the surface completely clean and ready to receive the primer before painting, In the case of DLC, the impact of a carbon atom on the surface induces pressure waves that locally change the density of the film and, in turn, orbital hybridization, that is, the type of chemical bonds that atoms can form in the film.

Roughness of the surface (left) and atomic structure of the DLC film (right). Source: APS Physics.
The team simulated the sequential deposition of individual carbon atoms on a carbon substrate. Using a previously developed automatic learning algorithm they calculated the interatomic potentials between the atoms and introduced that information into the molecular dynamics simulations. This procedure allowed them to map the density of the films after each impact. They discovered that the incoming atoms created a pressure wave that moved the atoms away from the impact site, so that each impact decreased the density of the atoms on the surface of the film and increased it within the film. Inside the film, the density change caused a high fraction of carbon atoms to acquire a tetrahedral hybridization: exactly the same state as the carbon atoms in the diamond. The simulated density profiles agree with those derived experimentally.
-- Katherine Wright, APS Physics.
DLC films are already used industrially as coatings for parts exposed to high friction. What these results can do is help chemists optimize the growth of DLC films, which can be extremely interesting for the construction of implantable electronic devices in the human body. And is that the DLC, being chemically inert, is biocompatible.
Reference:
Miguel A. Caro et al (2018) Growth Mechanism and Origin of High sp3 Content in Tetrahedral Amorphous Carbon Physical Review Letters doi: 10.1103/PhysRevLett.120.166101

Source
Copying/Pasting full or partial texts without adding anything original is frowned upon by the community. Repeated copy/paste posts could be considered spam. Spam is discouraged by the community, and may result in action from the cheetah bot.
More information and tips on sharing content.
If you believe this comment is in error, please contact us in #disputes on Discord
Hi @mofeta.
I have found a similar text here. You changed a few words but it is still plagiarism. Please refrain from doing that in the future. Read, understand and write it in your own words.
This post has received a 3.21% upvote from @lovejuice thanks to @mofeta. They love you, so does Aggroed. Please be sure to vote for Witnesses at https://steemit.com/~witnesses.
Beep bop, this is @pushbot.
I just received a signal from the Mother Ship that you may require a push.
You just got a 17.25% upvote courtesy of @mofeta!
Message from the Mother Ship:
You can earn daily profit by delegating SP to make @pushbot stronger. Delegators receive a share in 95% of the earnings.
10 SP • 20 SP • 50 SP
100 SP • 200 SP • 500 SP
1000 SP • 2000 SP • 5000 SP
Any Other Amount
You got a 11.05% upvote from @peace-bot courtesy of @mofeta!
Help spread the peace. Want to promote your posts too? Send a minimum of .02 SBD or STEEM to @peace-bot with link in the memo for an upvote on your post. You can also delegate to the bot for daily passive earnings. If you would like to delegate to the Peace Bot you can do so by clicking on the following links:
50SP 100SP 250SP 500SP 1000SP 5000SP
Learn more!
This post has received a 6.42% upvote from @msp-bidbot thanks to: @mofeta. Delegate SP to this public bot and get paid daily: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP Don't delegate so much that you have less than 50SP left on your account.
You got a 0.80% upvote from @oceanwhale With 35+ Bonus Upvotes courtesy of @mofeta! Delegate us Steem Power & get 100%daily rewards Payout! 20 SP, 50, 75, 100, 150, 200, 300, 500,1000 or Fill in any amount of SP Earn 1.25 SBD Per 1000 SP | Discord server