
From all the minerals I have known graphite is a very interesting and personal one for me. When I was in fourth grade I earned the nickname “Lead” by getting pencil lead all over myself when I used a pencil. Since pencil lead is made of graphite and clay little did I know at the time that my nickname derived from one of the most useful minerals we know of.
Graphite is a very useful and abundant mineral. We use it in pencil lead, batteries, and as a lubricant. It is very stable so it is very safe and consistent. It occurs naturally in large veins that can be mined underground. There are large commercial deposits of graphite in Ticonderoga, Essex Co., New York, and in Clay Co., Alabama.
The mineral graphite is a member of the group of minerals known as Native Elements. The Native Elements group are identified as having a molecular structure that contains only a single element. The single element contained in graphite is carbon.
Graphite is also classified as non-metallic. Minerals are considered non-metallic when they do not exhibit any metallic properties. However, there is one exception to graphite’s non-metallic nature, its metallic luster. Although graphite is classified as non-metallic, it does conduct electricity well.
Diamonds are also Native Elements composed exclusively of carbon. Diamonds are different from graphite only in their molecular structure. The molecules are arranged differently between graphite and diamonds. This differing arrangement makes diamonds and graphite have almost opposite characteristics in their physical properties.
What is graphite?
Graphite is composed entirely of carbon atoms. Each carbon atom bonds with three others in what is called a covalent bond. These bonds form hexagons in the form of sheets. A sheet of these atoms is called graphene. Since carbon has four electrons for sharing and shares only three in the covalent bonds in graphene one remains to loosely bond with an adjoining graphene sheet. These loose bonds and parallel sheets of graphene form graphite.
Each carbon atom is composed of six neutrons in its nucleus and six electrons in its shells. This makes carbon quite stable since it has no free atom to repel and dispose of and no free proton to attract and capture a free electron. This allows carbon to remain stable and not react or form ionic bonds when in contact with most other elements.
The carbon atom has two electrons in its inner shell and four electrons in its outer shell. To form graphite, first each carbon atom forms strong covalent bonds with three other atoms. This creates hexagons arranged end to end. The hexagon arrangements can extend for very long distances from the atomic perspective forming flat planes of sheets. These sheets of carbon atoms are called graphene.
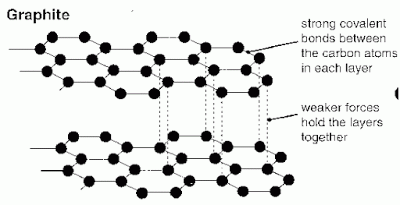
Next, the carbon atoms form loose and weak bonds with other atoms using their remaining forth atom in their outer shell. This bond adjoins the graphene sheets in a parallel arrangement. The graphene sheets become stacked forming layers of carbon sheets. These layers form the rocks and crystals known as graphite.
Uses of Graphite
Graphite has many uses. It is used in the manufacture of pencils. It is the black center of a pencil that is used to write with. Graphite is also an excellent lubricant. The weak bonding of the graphene layers allow them to slide across each other easily. For this reason it creates a slippery surface on objects it is applied to.
The pencil is composed of a hollow stick of wood with a thin stick of lead, which is a mixture of graphite and clay, in the center hollow. The process for making a pencil is quite interesting. There are many steps an actors in the making of a pencil. Here are the steps in the process of the manufacture of a pencil:

- At the Slat factory, pencil stock is cut into “Pencil Blocks”
- Pencil Blocks are cut into “Pencil Slats”
- Pencil Slats are treated with wax and stain to obtain uniform color and improve the machining and sharpening characteristics of the wood for future processing.
- At the Pencil Factory a “Groover machine” cuts grooves into the slats to accept the writing core (or “lead”).
- Writing cores – made from a mixture of graphite and clay – are placed into the grooves.
- A second grooved slat is glued onto the first – making a “sandwich”
- Once the glue dries, the sandwiches are transferred to a “Shaper”
- Individual pencils cut from the sandwich are ready for further processing.
- Next, each pencil is painted in a machine receiving from 4-10 coats of lacquer, depending on the desired quality of the finish and the color depth.
- On a “tipping” machine, an eraser and a ferrule (the metal ring that holds the eraser to the pencil) are crimped into place on each pencil (Pencils.com).
Another very common and widespread use of graphite is for lubrication. It performs excellently as a lubricant as long as moisture vapor is present. It remains a great lubricant up to 1,450 degrees Fahrenheit. Over those temperatures it continues to perform well as a release and anti-seize agent up to about 2,400 degrees Fahrenheit.

Graphite has the characteristics and properties of a metal and a nonmetal. This makes it extremely suited for many industrial applications. One metallic property of graphite is that it conducts heat well. Another metallic property is that it conducts electricity. One of its nonmetallic properties is that it is inert. Another nonmetallic property is that it is thermal resistive.
Because of graphite’s thermal resistive properties it has long been used in refractories to hold molten metal. It is also used in alumina-graphite shapes. Blocks are also used in parts of blast furnace linings where the high thermal conductivity of the graphite is critical. According to the USGS 11,000 tons of graphite were consumed by refractories in 2006.
Natural amorphous and fine flake graphite are used in brake linings or brake shoes for heavier (nonautomotive) vehicles, and became important with the need to substitute for asbestos. This use has been important for quite some time, but nonasbestos organic (NAO) compositions are beginning to cost graphite market share. A brake-lining industry shake-out with some plant closings has not helped either, nor has an indifferent automotive market. According to the USGS, US natural graphite consumption in brake linings was 6,510 tonnes in 2005.
Graphite Mining Locations
Graphite is found in many locations on earth. The countries that produce the most throughout the world are China, India, and Brazil. Some of the largest deposits in the United States have been found in Ticonderoga, Essex Co., New York, and in Clay Co., Alabama.
According to the Alabama Graphite Company the Coosa Graphite Project is the most advanced flake graphite project in the contiguous United States of America. According to their website (http://alabamagraphite.com/company-overview/):
The Coosa Graphite Project hosts an NI 43-101 Indicated Mineral Resource Estimate of 78.5 million tons, grading 2.39 % Cg — the largest Indicated Mineral Resource Estimate of flake graphite in the United States of America.
Recent metallurgical testing of Coosa graphite concentrate resulted in purities of 99.99% Cg — the threshold for battery-grade graphite is 99.95% Cg.

I love it when graphite gets stuck under my skin after someone stabs me with a #2 pencil. Makes me feel like I'm #1. :)
LOL. I feel that
In powder form it is a great lubricant for zippers. Just get a no. 2 pencil and scrape the lead across a zipper. It will zip better!