
In the first part we have known about the stability of the star which is supported by the force of radiation pressure and the force of gravity. Furthermore, in the second part, we follow a process of thinking that concludes that radiant energy is produced from radioactive processes and that the observation of the solar light spectrum throughout the 19th century shows that the Sun is full of Hydrogen.
Norman Lockyer discovered a mysterious element in the Sun, an element not found on Earth
In 1868, almost simultaneously, French astronomer Pierre Janssen and British astronomer Norman Lockyer observed a mysterious element in the Sun. An element not found on Earth. Lockyer then named this mysterious element is Helium, from the Greek word "Helios" which means the Sun.
Only about 30 years later in 1895, Scottish chemist William Ramsay accidentally discovered Helium gas on Earth. Ramsay burns sulfuric acid to search for Argon, but after separating the Nitrogen and Oxygen gas created from the combustion, Ramsay sees the spectrum of the mysterious element of the Helium. Together, Hydrogen and Helium are generally the two most abundant elements in a star. Our sun, for example, contains 34% Hydrogen and 64% Helium, and 2% is a combination of other elements.
The centuries-old secret of the compiler of the Sun has been answered. When astronomers direct their spectroscopes toward other stars, another mystery unfolds about the nature of stars: the spectrum of stars is the same as the Sun! In other words, the Sun is a star that is very close to us. Stars and Suns are the same object but the star distance is much greater than the distance of our Earth to the Sun. The magnitude of the role of spectroscopy in uncovering the secrets of nature is then remembered by parodying the text of Small Star songs in English:
I don’t wonder what you are;
For by spectroscopic ken,
I know that you’re hydrogen;
Twinkle Twinkle little star,
I don’t wonder what you are.
Why the sun and stars can shine? Where is the energy? Research in the late nineteenth and early twentieth centuries about the nature of atoms and radioactivity concludes that it is the nuclear reaction that generates the energy of the Sun. In the second part, we have seen that the abundant Hydrogen in the Sun can carry on a nuclear reaction for billions of years. What does this nuclear reaction look like?
Fusion reactions can occur in extreme conditions, and it has been estimated that the Sun's core is extreme enough to carry out such reactions. As we know, the temperature at the core of the Sun is about 15 million Kelvins. In the theory of gas dynamics, the temperature of a gas denotes the kinetic energy contained in the gas, due to the atomic motions of the gas. The extremely high temperatures in a gase state the remarkable atomic movement. Extremely high pressures may also state the density of the gas. The denser the gas, the closer the atomic nucleus is to each other.
To trigger a fusion reaction, two atoms must be able to overcome the reject force between the two. The nucleus of the atom has a positive charge that repels each other when it meets a similar charge. As a result, two Hydrogen atoms that are reunited will reject each other. This rejection force will get bigger as the distance gets closer. But if the distance between these two atoms is so close then the tensile force called the strong nuclear force can overcome the forces of repulsion between the two nuclei, binding the two nuclei of Hydrogen and be forming Helium. What is the minimum distance that two Hydrogen atoms must be able to melt into Helium?
Armed with nuclear physics knowledge, Fritz Houtermans tries to answer this question. He was born in Zoppod, a small town near Danzig in Baltic Germany (now named Gdansk and is in Poland). In the 1920s he worked as a researcher at Gottingen, Germany, and collaborated with British researcher Robert d'Escourt Atkinson to explain the nuclear reaction in the Sun. Together, they calculate that the minimum distance that the two atoms must reach is 10-15 meters or one by one trillion millimeters (!) They are convinced that the gas density at the center of the Sun is so high that the distance between atoms will be very close, and even more energy the kinetic will be so high that their movement will be very fast. It is likely that there will be atoms that can reach this small distance and trigger a nuclear reaction. The results of their calculations were published in the scientific journal Zeitschrift für Physik in 1929. So happy was Houtermans with their calculations, so in the afternoon he boasted his findings on the girl he dated. That night, the stars shone brightly and his girlfriend said, "how beautiful are those stars?" Houtermans replied, "Since yesterday I already knew what caused them to shine." Charlotte Riefenstahl, the girl was amazed, and then married her.
Houtermans may be proud of themselves, but there is still a problem with its findings regarding minimum distances that can trigger fusion reactions. At this critical distance, the magnitude of the potential energy generated by the two atoms is about 1000 kilos of Volt electrons. If an atom that has reached this critical distance does not have more energy than this energy, then the melting will not occur. So there is a potential "wall" that a Hydrogen atom must penetrate when it wants to fuse with other Hydrogen atoms. However, every average Hydrogen atom has an only energy of 1 keV, 1000 times less than the critical energy that must be penetrated. According to statistics, a small percentage of particles have the same or even greater energy than this critical energy. However, the amount of these high-energy particles is so small that the nuclear reactions that occur will not be large enough to last for billions of years. How do we answer this problem?
Quantum theory saves this problem by offering a different perspective in physics. If 18th-century physics is so deterministic as to say that the position of a particle can be known from time-to-time, then quantum theory says that we can only know the probability of finding a particle in a particular location. On a small scale in the particle world, the position of a particle is not at all sure. It can be anywhere and all we can determine is the likelihood that it will be in a location. Armed with this worldview, the Ukrainian-born physicist George Gamow solved this potential problem through the phenomenon he called "the quantum tunnel effect." From the perspective of quantum physics, we can calculate the probability of finding a particle within that critical distance, and thereby be melting and initiating nuclear reactions. This opportunity increases with the higher energy of the particle, and by comparing it with the energy distribution of a particle collection, it can be calculated the range of energies in which nuclear reactions are most likely to occur. This change of perspective allows us to solve the problem of energy generation in stars. Gamow, a Soviet physicist who later fled to the United States, thought of the tunnel effect to explain the decay phenomenon in the perspective of quantum physics. However, it is known that the tunnel effect is also applicable in general and can be used also to explain the opposite phenomenon of joining atomic nuclei.
Houtermans's work on nuclear reactions in the star was then continued by Hans Bethe. Born in Straßburg, Germany (then Strasbourg and entered France) in 1906, Bethe obtained his doctorate from the University of Munich, Germany, under the guidance of Arnold Sommerfeld. After working in Cambridge and in Rome with Enrico Fermi, Bethe taught at the University of Tübingen until 1933. At that time the Nazi Party came to power and Bethe was fired from his job because his mother was Jewish. Bethe moved to England and in 1935 moved to the United States. Together with many other nuclear physicists, Bethe then worked on developing an atomic bomb at the Los Alamos Laboratory and headed the Theoretical Division.
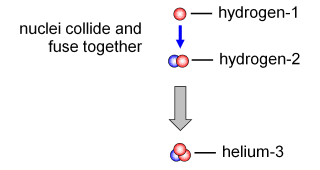
Bethe's work on nuclear physics enabled him to identify fusion-reaction pathways that enabled the creation of a stable Helium nucleus. The atom of an element has a variety of types called isotopes. What distinguishes the isotopes of an element from another is the number of neutrons contained within the nucleus. Neutral Hydrogen or Protium, for example, has 1 proton and 1 electron. Deuterium, one of the isotopes of Hydrogen, has an additional 1 neutron and is relatively stable. Helium-3 and Helium-4 are two of the 8 stable isotopes of Helium atoms, each having 1 and 2 neutrons at their core. Houtermans expects that the star's fusion reaction takes place through the incorporation of two neutral Hydrogen nuclei into Diproton, a very light and unstable Helium isotope. Two neutrons are needed to create stable Helium isotopes, but by the time Houtermans and Atkinson wrote their paper in 1929, the existence of neutrons was still a hypothesis. As a result, the calculation of Houtermans is not yet complete.
As Bethe continues the work of Houtermans, our picture of the atomic world is more complete. Two neutral Hydrogen atoms can melt first to form Deuterium. Furthermore, Bethe sees this Deuterium can capture 1 other neutral Hydrogen atom to form relatively stable Helium-3. These two Helium-3s can then melt to form a more stable and non-radioactive Helium-4. As a by-product, two Hydrogen atoms will be released. This reaction is then known as the Proton-Proton Reaction or PP Reaction because it all starts with two protons that melt away.

Proton-Proton reactions can still be continued into PP-II Reactions. Helium-3 and Helium-4 can melt to form Beryllium-7 which can capture an electron to become stable lithium-7. Furthermore, Lithium-7 can capture a Hydrogen atom and transform into 2 Helium-4 atoms. This occurs when the core temperature is between 14 to 23 Million Kelvin. At core temperatures above 23 Kelvin, PP-III reactions occur: Beryllium-7 will capture neutral Hydrogen and transform into Boron-8. Because Boron-8 is unstable, it will decay into Beryllium-8, which in turn will decay into 2 Helium atoms.
In addition to the PP Reaction, Bethe also proposed another route to creating another route that uses Carbon atoms as a trigger that serves to capture Hydrogen atoms. If in the core of the Sun there is Carbon-12, then each Carbon-12 nucleus will be able to capture Hydrogen to form nuclei of heavier atoms, ie Nitrogen, and Oxygen respectively. Nitrogen-15 (see figure) is unstable and will merge again into Carbon-12 and will again capture a Hydrogen atom to start this cycle back to the beginning. Because these chain reactions form a cycle, then this series of reactions is called the Carbon Cycle or Cycle.

At first these two nuclear reactions are still speculative. Other physicists then examine Bethe's calculations and ensure that this reaction can occur when conditions are right.
In the 1940s it was clear that these core reactions did indeed occur in the "furnace" of the Sun. Observation of the solar spectrum is again the key because the abundance of chemical elements generated from these reactions can be confirmed through the Sun's spectroscopy. For Bethe's services of identifying the energy production of the stars, he was rewarded with the Nobel Prize in 1967.
After looking at the forms of the PP Reaction and Carbon Cycle, we may see that this reaction essentially converts Hydrogen into Helium. Slowly but surely, Hydrogen is transformed into Helium and can run out. In the end, if a star can no longer burn Hydrogen into Helium, then another way to generate energy that can offset the pressure of gravity must occur. If it does not exist, then the star will not be able to withstand the pressure of gravity and will collapse. Is there still another way?
Two Helium-4 atoms can combine to form Beryllium-8, which in turn can capture another Helium-4 atom to become Carbon-12. This reaction is crucial because it is the only nuclear reaction that can create significant amounts of Carbon in the universe. But many problems that block this reaction can occur. This reaction can only occur at extremely high temperatures, ie, at 100 Million Kelvin. Another requirement to occur is when there is a large number of Helium-4 atoms. The next problem is Beryllium-8 is a very unstable atom and can only survive in less than 10-18 seconds or just one billion-billionth of a second, very very short! Almost impossible Beryllium-8-before its decay -can catch the nearest Helium-4 to turn into Carbon-12. Even if this can happen anyway, there are other obstacles to be faced.

The combined mass of Helium-4 with Beryllium-8 is larger than the Carbon-12 mass, so if both atoms can join, there will be excess mass to be discarded. Of course, the excess mass will be converted into energy through the equation E = mc2, but the greater the mass difference the reaction time will be longer and Beryllium-8, whose decay time is very fast, do not have time to wait for this reaction is completed. Carbon-12 must be formed immediately because the age of Beryllium-8 is very, very short.
Carbon is the most abundant element in the universe after Hydrogen, Helium, and Oxygen. George Gamow and his guidance student Ralph Alpher found that within a few minutes after the big bang, the universe comprised 75% of Hydrogen and 25% of Helium, but the heavier elements were not created because the universe had cooled down before it happened fusion reactions that allow the formation of heavy elements. But in fact, on this Earth we find heavy elements, ranging from Hydrogen, Helium, Lithium, to Uranium, Plutonium, and so on. On our Earth, heavy elements like Silicon, Aluminum, Iron, are the most abundant elements. The human body contains 18.5% Carbon and we know Carbon is an element that is always present in almost every form of life. Answering the question of the origin of this heavy element is equivalent to answering some questions about the origin of life, a question that the human civilization continually asks.
To explain the formation of the heavy elements in the universe, Fred Hoyle, the British astrophysicist, created a Triple-Alpha reaction. He found that the only way to create Carbon was through nuclear reactions in the realm of star cores that were unusually hot and filled with Helium. But this reaction, if it can happen, is very problematic. First, Beryllium-8 is very unstable and can not last long. Secondly, the change of Helium and Beryllium into Carbon takes a significant time due to massive differences in mass. There seems to be no solution to this situation, but Hoyle is able to finish it brilliantly. Hoyle's process of mind in addressing this issue will be the next topic.
Reference :
- http://hyperphysics.phy-astr.gsu.edu/hbase/Astro/astfus.html
- https://en.wikipedia.org/wiki/Nuclear_fusion
- https://en.wikipedia.org/wiki/Norman_Lockyer
- https://en.wikipedia.org/wiki/William_Ramsay
- https://en.wikipedia.org/wiki/Fritz_Houtermans
- https://en.wikipedia.org/wiki/Electronvolt
- http://www.phy6.org/stargaze/Q8.htm
- https://en.wikipedia.org/wiki/Hans_Bethe
- https://en.wikipedia.org/wiki/Isotopes_of_hydrogen#Hydrogen-1_(protium)
- https://en.wikipedia.org/wiki/Deuterium
- http://hyperphysics.phy-astr.gsu.edu/hbase/Astro/procyc.html
- https://en.wikipedia.org/wiki/CNO_cycle
- https://physics.aps.org/articles/v4/38




Congratulations! This post has been upvoted from the communal account, @minnowsupport, by whalhesa from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.