France produces 75 per cent of its electrical power from nuclear power stations and only 12 per cent from fossil fuels. This compares with just 28 per cent nuclear power in Britain, and 70 per cent from fossil fuels.source Fossil fuels contribute to the greenhouse effect and global warming. They also pollute the atmosphere, and most developed countries are searching for ways to reduce their dependence on fossil fuels. This suggests that making more use of nuclear power should be a good thing.
But in the United States (the world’s largest producer of electricity from nuclear power), electricity suppliers decided in 1987 not to build any more nuclear power stations. Britain, too, cancelled plans in 1995 for building two large nuclear power stations. The main reason for these decisions was economic. In the debate on energy from nuclear or fossil fuel sources, economic and environmental arguments compete. Since then, politicians have had to think harder about how we use and obtain energy. Looming on the not too distant horizon is global warming. The pollutants produced by burning fossil fuels make it easier for the atmosphere to trap solar thermal radiation. The main culprit seems to be carbon dioxide.
At the atomic level, the thermal energy we can get from a nuclear fission event is 200 MeV, compared with just a few eV from breaking up a molecule of a carbon-based fuel. It takes 1.5 tonnes of coal to produce as much energy as we can get from a piece of nuclear fuel about 15 mm long. For the UK, North Sea gas and oil are running out, so they are becoming more dependent upon foreign suppliers of fossil fuel.
Chernobyl Nuclear Power Station was badly designed and badly maintained. The first generation nuclear power stations were designed in the 1950s – they are now building Generation 3 power stations in Finland and in France, and Generation 4 designs are in the pipeline. The main benefits from these new designs are much-increased efficiency for safer operation and much less radioactive waste.
People are scared of anything called nuclear, and of radiation. The utterly safe and effective medical diagnostic tool Magnetic Resonance Imaging (MRI) had to change its original name of Nuclear Magnetic Resonance (NMR) because of the N-word. People, in general, don’t realise that the dangerous stuff is ionising radiation. You can sit in front of an electric fire without coming to much harm.
The ideas in this post
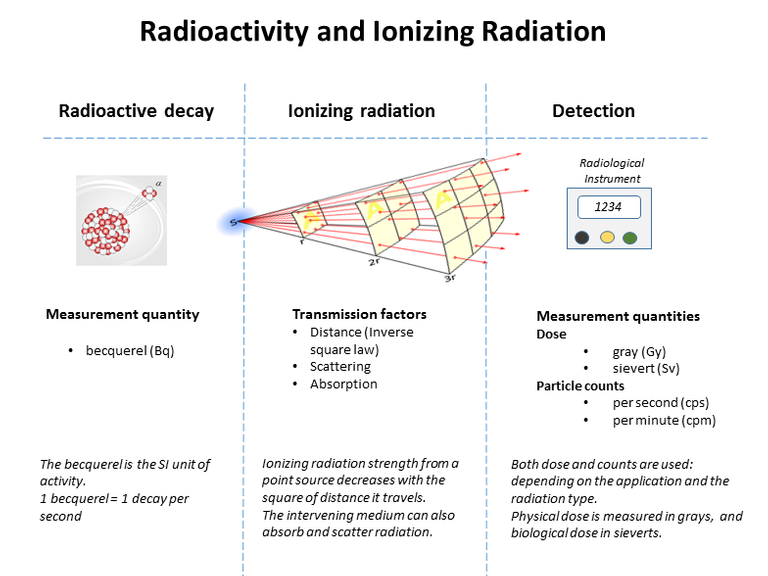
The atomic nucleus was not known about until the early twentieth century. Yet, only thirty years on, in 1945, two nuclear bombs were to kill an estimated 200 000 people in Japan, an event which ended the Second World War in a matter of days. This chapter deals with the evidence we have about the nature and structure of the nucleus, and why some nuclei break up and others are radioactive. It was the chance discovery of radioactivity at the end of the nineteenth century that began the series of investigations that led to an understanding of what an atom was like: a tiny but massive nucleus surrounded by a cloud of electrons. The evidence came from using the emissions that came from unstable nuclei: alpha, beta and gamma radiations.
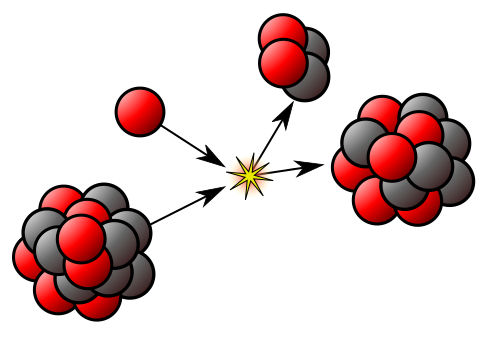
The nucleus can provide useful energy through a process that occurs only with certain rare, very large nuclei, when they break up into two smaller nuclei – nuclear fission. Radioactivity itself is a minor source of energy, but the radiations are useful tools for industrial and medical use. The radiations are able to knock electrons from, or ionise, ordinary atoms. The ionisation effects allow the radiation to be easily detected – but also cause harm to living tissue.
Life on Earth depends on the energy from nuclear reactions in the Sun known as nuclear fusion, in which two hydrogen nuclei combine to form a helium nucleus. But attempts to use nuclear fusion as a steady, controlled source of cheap and almost pollution-free energy have so far been unsuccessful.
RADIOACTIVITY
Radioactivity was discovered in 1896 by Henri Becquerel (France, 1852-1908). He was investigating the property that some natural minerals have of glowing in the dark – fluorescence. He detected the faint light emitted by fluorescent substances using photographic plates. One day he found (by accident) that a salt of uranium emitted invisible radiation which blackened the photographic plates.
Soon, three types of radiation were discovered, and because they were then unidentified they were simply labelled alpha (α), beta (β) and gamma (γ) rays, after the first three letters of the Greek alphabet. The table below lists the nature and properties of the radiations.
Properties of alpha, beta and gamma radiations
| Radiation | Alpha | Beta | Gamma |
| Nature | helium nucleus (2n + 2p) | electron or positron | photon of electromagnetic radiation |
| Symbol | Нe | e | γ |
| Charge | 2e+ | e- or e+ | γ |
| Range in air | 1-5 cm | 10-100 cm | infinite – obeys the inverse square law |
| Range in matter | stopped by e.g. a sheet of paper | stopped by e.g. a thin (1 mm) sheet of aluminium | intensity reduced to half by e.g. 1 or 2 cm of lead |
| Mass in atomic mass units | 4 u | 5 × 10-4 u | zero |
| Relative ionising ability | very high: 1 | high: 1/100 | low: 1/10000 |
Marie Curie (Poland/France, 1867-1934) and her husband Pierre (France, 1859-1906) investigated radioactivity and developed ways for measuring the activity of sources, i.e. how much radiation a source emitted per second. In doing so they discovered other elements that also emitted energetic radiation, identifying completely new ones such as polonium and radium. Later, the physicist Ernest Rutherford (New Zealand/Britain, 1871-1937) and the chemist Frederick Soddy (Britain, 1877-1956) worked together to establish the nature of the radiations and track down the changes of the elements that were linked to them. Their results began to make sense when Rutherford’s team discovered the nucleus in the years 1909-1911.
DETECTING THE RADIATIONS
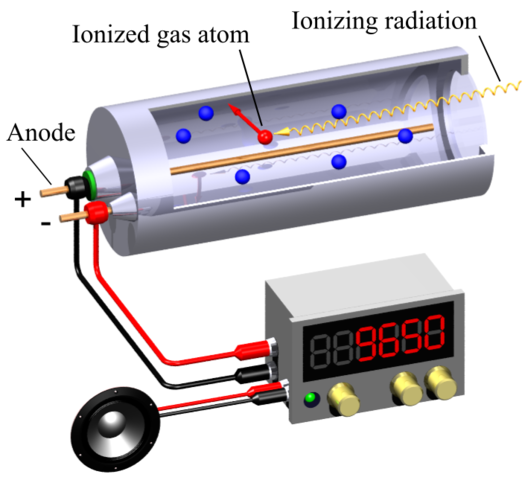
THE GEIGER COUNTER: The most common device – and one of the oldest – for detecting and monitoring ionising radiation is the Geiger counter. The key part of the counter is the Geiger-Müller tube, the GM tube. A sealed metal tube has a thin wire down the middle and is filled with a non-reactive gas (e.g. argon) at low pressure. The wire is kept at a voltage of about +400 V with respect to the metal case, which is earthed. The radiation enters the tube through a thin window in the front. Alpha particles are easily absorbed by matter so the window in an alpha detector is a thin film of mica – but strong enough to withstand the pressure difference between the atmosphere and the gas inside the tube.

Geiger-Müller radiation detector. Boffy b, CC BY-SA 3.0
Radiation entering the tube ionises argon atoms. The positive argon ions are attracted to the metal case and electrons to the central wire. These charged particles are accelerated by the voltage and will collide with other argon atoms, If they are moving fast enough, the collisions will ionise the atoms. The new particles are in turn accelerated and will cause further ionisation, producing an avalanche effect. The sudden large flow of charge in the tube causes a pulse of current in the central wire which is amplified electronically.
The amplified pulses may be counted by a scaler or averaged out to give a current reading on a ratemeter. The pulses may also be fed into an audio circuit to give a series of clicks, so providing an audible indication of the radiation level. The charge avalanche takes tìme to be cleared out of the gas, so there is a dead time (of about 200 ms) in which the tube cannot react to any incoming radiation. Beta and gamma detectors are similar: windows for beta detectors can be made of glass, and gamma detectors can be made entirely of metal.
Working voltage of a GM tube
The voltage on the central rod has to be set high enough to accelerate ions and electrons and cause the avalanche effect. But the voltage should not be so high as to produce a continuous discharge. This happens when a continuous supply of ions hitting the metal walls have enough energy to eject electrons from it, which are then accelerated to cause further ionisation of the argon atoms. There is a plot of the count rate, which is proportional to the mean current in a GM tube, against voltage when the ionising radiation enters the tube at a constant rate. The operating plateau shows the range of applied voltage for which the tube works well. At plateau voltages, all ionising particles of the same type will produce the same size pulse of charge.
SOLID STATE DETECTORS
In industry and research laboratories, the most common kind of radiation detector is ‘solid-state’. The energy of the incoming radiation (particle or photon) liberates electrons from semiconductor material. This produces a pulse of current and so indicates the arrival of the radiation. Such detectors can both count individual events and also measure the energy of the radiation.
PHOTOGRAPHIC DETECTION
Photography works because photons of light have an ionising effect on certain chemicals – generally salts of silver such as silver nitrate. Radiation badges, use this effect. Such a badge has to be worn by anyone who works in an area where they might be exposed to ionising radiation, such as a nuclear power plant, an X-ray department in a hospital, or a radioactivity laboratory. More advanced particle detectors will be described later.
BACKGROUND RADIATION
A Geiger counter set up anywhere on Earth will always register a count. This count is due to background radiation, produced by tiny fragments of radioactive elements present in all rocks and soil, the atmosphere and even in living material itself. Also, the Earth is continuously bombarded by high-speed particles from outer space and from the Sun called cosmic rays. Cosmic rays smash up the nuclei of atmospheric molecules and produce other high-speed particles which can cause ionisation. Many of these reach ground level.
REFERENCES
https://www.world-nuclear.org/information-library/country-profiles/countries-a-f/france.aspx
https://en.wikipedia.org/wiki/Nuclear_power_phase-out
https://en.wikipedia.org/wiki/Olkiluoto_Nuclear_Power_Plant
https://en.wikipedia.org/wiki/Chernobyl_Nuclear_Power_Plant
https://www.britannica.com/science/atomic-nucleus
https://simple.wikipedia.org/wiki/Atomic_nucleus
http://www.radioactivity.eu.com/site/pages/Atomic_Nucleus.htm
https://en.wikipedia.org/wiki/Atomic_nucleus
https://en.wikipedia.org/wiki/Radioactive_decay
http://www.physics.org/article-questions.asp?id=71
https://www.britannica.com/science/radioactivity
https://www.nrc.gov/about-nrc/radiation/health-effects/detection-radiation.html
https://hps.org/publicinformation/ate/faqs/radiationdetection.html
https://www.quora.com/What-is-the-operating-voltage-in-a-GM-counter-and-how-is-it-obtained
http://scientificsentence.net/Phys_Meas/index.php?key=yes&Integer=Geiger
https://en.wikipedia.org/wiki/Geiger%E2%80%93M%C3%BCller_tube
https://www.imagesco.com/geiger/geiger-counter-tubes.html
https://www.sciencedirect.com/topics/mathematics/solid-state-detectors

I appreciate this thoughtful post. In the spirit of dialogue, may I suggest that in providing energy supply we do not have a binary choice. It is not either fossil fuels or nuclear. All the brilliant minds that have developed and refined nuclear energy should apply their skills to at least two more options: clean energy (solar/wind/hydro) and highly efficient technology that uses less energy.
Of course, there is no truly 'clean' energy, if we look at the cost (environmentally) of manufacture and disposal of energy components. Ideally, we will learn to live less wastefully and use less energy, overall.
Many of us who oppose nuclear do not have an instinctive aversion to the word 'nuclear'. We have an aversion to the unavoidable and virtually immortal radioactive waste inherent in nuclear energy production. We have an aversion to the admittedly remote but still present danger of catastrophic failure. And that failure can arise from events not intrinsic to the plant's operation. For example, NY's Indian Point nuclear reactor was built very near to the Ramapo Fault line. We all know about Fukushima. And then there's the ongoing danger of deliberate sabotage by bad actors.
We don't have to choose between dirty fossil fuel and implicitly dangerous (yes, dangerous) nuclear. We can use the vast resources of industry and government to come up with a saner option...energy efficient technology and renewable energy.
Hope you don't mind my long comment. This is not a simple discussion and it is one to which I have given some thought. I look forward to reading more of your always interesting, and informative, posts :)
I am fully in line with you! The problematics is much larger than naively thought of.
Happy new decade by the way :)
Hi @lemouth, Happy New Year!
I missed you. You're back, with another blog to challenge me and bring me into the wider universe :)
I'm glad you agree with me. I've done a lot of reading, careful reading, and can find no excuse (except immediate financial interests) for continuing down the nuclear path. You certainly have a better understanding of the technology then I do, but this is not something that should be left to specialists. We all live with the consequences.
Anyway, so glad you're back. Hope your little ones had a joyous holiday.
I am rather partly back, but I will try to blog about particle physics again soon :)
IMO, the reasons for using nuclear energy is not only financial (usually, the same companies, at least in France, own several types of plants). It is a choice between polluting in one way or the other (and there is no good choice... at least for now).
There is in fact no need to looking for excuse. All available technologies of today have pros and cons and decisions have to be made. To me, the error is that not enough money is put in research for future, cleaner and more efficient, options.
Just to comment to the last part of your message, I am a bit rusted in how this technology works as my student days in nuclear engineering are far far behind me, so that I am maybe not 100% aware of the new developments and I forgot many things. I have actually never even started with working in this field as I switched gears to particle physics research right after my studies.
Cheers!
PS: I had a few days of offline break between Xmas and new year. This was good!
I fully agree with @agmoore2! Currently, nuclear power is the cleanest source of energy in terms of greenhouse gas emission. It is even cleaner than wind or solar power when all the cycle is accounted for. However, there is the problematics of the waste.
The choice stays binary if we do not account for future developments. In my opinion, we should do more research on what to do with the nuclear waste (that also comes from other source than just the energy industry, by the way) and improve the existing technology. On the other hand, it is very important to further develop clean energy sources. By only working out these two together, we may open the door to new (potentially preferred) options.
On the other hand, we as human beings waste a lot of energy. We should be more careful with our planet and use its resources more carefully.
@tipu curate
Upvoted 👌 (Mana: 5/10 - need recharge?)
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.
Congratulations @emperorhassy! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOP