Perkin and the beginning of the dye industry
William Perkin was the son of a carpenter in East London. As William had some artistic talent, his father hoped his son would train as an architect. However, a fascination with chemistry led him to become a student at the Royal College of Chemistry. At the age of 18 years he made an accidental discovery that was to prove to be the start of not only the dyestuffs industry but also the whole organic chemicals industry.
 )
[Pixabay, silviarita; CC0](https://pixabay.com/photos/egg-easter-food-color-background-3165476/)
)
[Pixabay, silviarita; CC0](https://pixabay.com/photos/egg-easter-food-color-background-3165476/)
At the Royal College of Chemistry, Perkin became an assistant of Professor August von Hofmann, a German chemist. Hofmann suggested that he might like to try synthesise the important, naturally occurring drug quinine, which was used to treat malaria. The starting material was coal tar, because it was known to contain arylamines. At this time, the structures of organic chemicals had not been deduced.
However, the molecular formula of quinine was known, as was the empirical formula of the arylamine he intended to use. Perkin thought that if he oxidised the arylamine he would produce the reaction:
2C10H13N + 3[O] ― C20H24N2O2[quinine] + H2O
All Perkin managed to produce was a dirty brown precipitate. Undeterred, he set about oxidising a simpler compound, phenylamine. This time he produced a black precipitate that, when dried and dissolved in ethanol, produced a brilliant purple solution. The product's structure was nothing like that of quinine, but Perkin was quick to see its potential as a dye. He dyed some silk with the purple dye and sent it to a firm of dyers.
The reply came back, 'If your discovery does not make the goods too expensive, it is decidedly one of the most valuable that has come out for a very long time.’ One of the most important properties of the dye was that it was fast and didn't fade or change colour, even when exposed to light and air. Perkin named the dye
“mauve” after a French flower.
 )
Mauve coloured flower
[Pixabay, Buntysmum; CC0](https://pixabay.com/photos/azalea-mauve-flower-2876219/)
)
Mauve coloured flower
[Pixabay, Buntysmum; CC0](https://pixabay.com/photos/azalea-mauve-flower-2876219/)
He left the Royal College of Chemistry and, with the help of his father and brother, set about building a factory for the large-scale production of mauve. Perkin used coal tar as the starting material because it was a cheap, plentiful by-product of the coal gas industry. He produced benzene by fractionally distilling coal tar. He then nitrated the benzene to produce nitrobenzene. The nitrobenzene was reduced using a mixture of iron filings and ethanoic acid to form phenylamine. This phenylamine he then oxidized using acidified potassium dichromate(VI).
Queen Victoria wore a dress dyed with mauve to the International Exhibition of 1862. Punch, the satirical magazine, proclaimed that policeman could be heard telling people to 'get a mauve on! It was not long before other dyes of different colours were made using phenylamine. Although the synthesis of dyes began in England, Germany soon became the centre of the dyestuffs industry.
Perkin's mauve was superseded because it cost too much to produce. One of its last applications was in the production of a 1d stamp. The stamp was printed in 1881, and was dyed by Perkin’s mauve. At 36 years of age, Perkin sold his factory and returned to his first love, chemistry research. He made many important contributions to organic chemistry, including the production of a perfume from coal tar. However, the synthesis of the quinine molecule was not finally achieved until 1944, almost 90 years after Perkin's attempt.
AN EXPOSITION ON DIAZONIUM COMPOUNDS AND AZO DYES
As I’ve narrated above, the first synthetic dye, mauve, was discovered in 1856 by an 18year-old Englishman, William Perkin. Perkin's discovery led to a search for new dyes and prompted the start of the organic chemical industry and one of the chemicals Perkin used in the production of the dye was phenylamine.
 )
Mechanism for the diazo coupling of benzenediazonium chloride with phenol, forming 4-(phenyldiazenyl)phenol
[Wikimedia, Ben Mills, public domain](
)
Mechanism for the diazo coupling of benzenediazonium chloride with phenol, forming 4-(phenyldiazenyl)phenol
[Wikimedia, Ben Mills, public domain]( )
)
Phenylamine is an aromatic amine (arylamine). It has a benzene ring with an NH
2, (amino) functional group directly attached to it.
 )
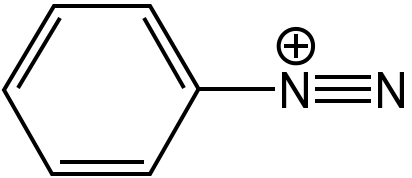
Diazonium molecular strucuture.
[Wikimedia, Javitomad; CC BY-SA 4.0](
)
Diazonium molecular strucuture.
[Wikimedia, Javitomad; CC BY-SA 4.0]( )
)
LET’S START WITH THE REACTION CALLED DIAZOTISATION
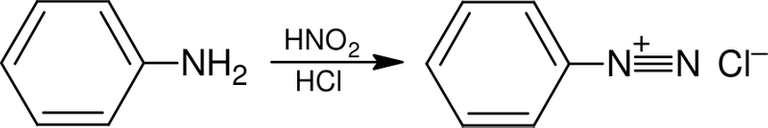
Six years after the discovery of mauve, Johann Peter Griess used aromatic amines to produce diazonium compounds (also known as diazonium salts). Diazonium compounds are produced when nitrous acid, HNO
2, reacts with an aromatic amine. The reaction mixture must be kept below 5°C because the diazonium salt is unstable. Nitrous acid is also unstable and it has to be made
[in situ](https://en.m.wikipedia.org/wiki/In_situ) by reacting sodium nitrite with concentrated hydrochloric acid.
NaNO2(aq) + HCl(aq) ― HNO2(aq) + NaCl(aq)
The nitrous acid reacts with the aryl amine and more of the concentrated hydrochloric acid to give the diazonium salt.
 )
diazonium salt
[Wikimedia; public domain](
)
diazonium salt
[Wikimedia; public domain]( )
)
It is this reaction that’s called diazotisation. The usual way to carry this out in the laboratory is to add a cold aqueous solution of sodium nitrite to a solution of phenylamine dissolved in concentrated hydrochloric acid. The reaction mixture is kept below 5°C by using ice.
The benzene ring stabilises the diazonium ion by delocalisation of its electrons. Alkylamines, such as ethylamine (C
2H
5NH
2), produce diazonium salts that decompose immediately, even below 5°C. Above 5 °C, the solution of benzenediazonium compound rapidly decomposes, giving off nitrogen gas according to this equation:
C6H5N2+ Cl-+ H2O ― C6H5OH + N2 + HCl
THE PRODUCTION OF AZO DYES USING COUPLING REACTIONS
A diazonium ion may act as an electrophile and attack the benzene ring of another compound. This reaction is called a coupling reaction. The first coupling reaction was observed by Griess: an aromatic amine coupled with a diazonium salt to produce a stable azo compound.
 )
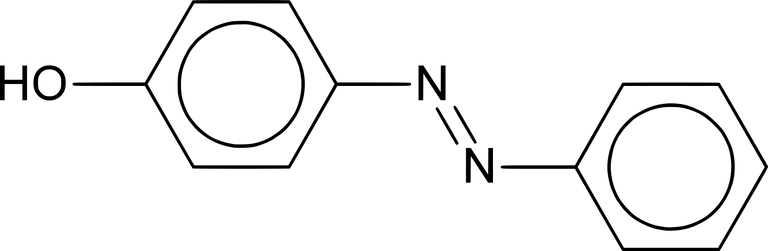
A typical azo compound: 4-hydroxyphenylazobenzene aka yellow azo dye
[Wikimedia, Ragimiri; public domain](
)
A typical azo compound: 4-hydroxyphenylazobenzene aka yellow azo dye
[Wikimedia, Ragimiri; public domain]( )
)
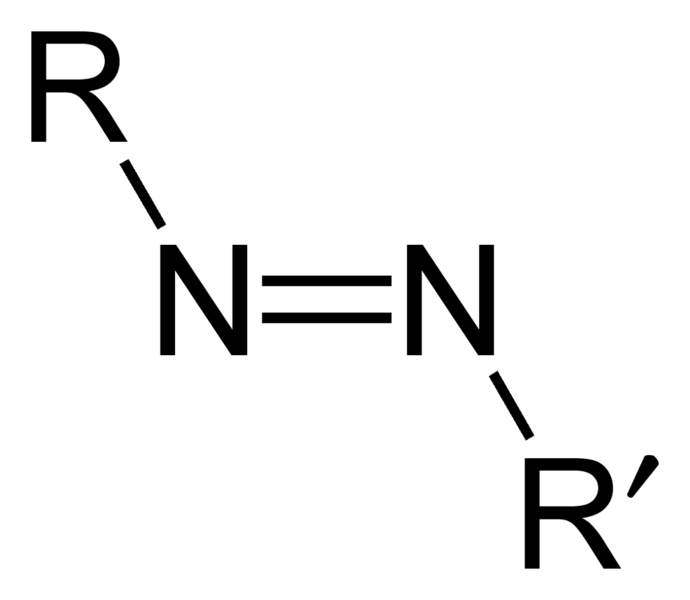
In the 1870s, coupling reactions involving alkaline solutions of phenols were first observed. The functional group of an azo compound is the azo group, ―N=N―. In a compound R―N=N―R', if the R groups are aromatic (aryl) groups, the azo group becomes part of the delocalised systems of the adjoining benzene rings. This gives extra stability to the molecule and is also responsible for the bright colours of azo dyes.
 )
Structural formula of the azo group, R-N=N-R′.
[Wikimedia, Benjah-bmm27; public domain](
)
Structural formula of the azo group, R-N=N-R′.
[Wikimedia, Benjah-bmm27; public domain]( )
)
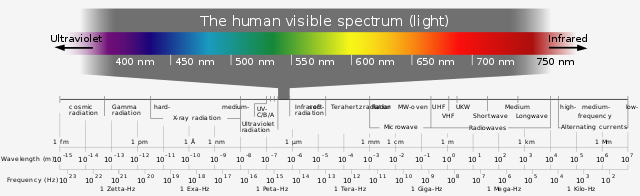
COLOUR IN ORGANIC MOLECULES
What gives rise to the bright colours of azo dyes? I have already explained in my first post on the chemistry of colour that colour results from electron transitions. If a substance absorbs electromagnetic radiation with frequencies in the visible spectrum, then it will be coloured. This is because the substance reflects or transmits only part of the visible spectrum.
 )
Electromagnetic Wave Spectrum
[Wikimedia, Horst Frank; CC BY-SA 4.0](
)
Electromagnetic Wave Spectrum
[Wikimedia, Horst Frank; CC BY-SA 4.0]( )
)
The absorption of a photon (a quantum of electromagnetic radiation) causes an electron to jump from its ground state (the lowest possible energy level) to an excited state (a higher energy level). If the difference in energy between the ground state and the excited state is equivalent to a photon with a frequency (or wavelength) in the visible part of the spectrum, the substance will absorb at that frequency when white light falls on it. Only outer shell electrons are excited by visible and ultraviolet radiation. Electrons from inner shells are held much more firmly by the nucleus, so they require much more energy to become excited.
REFERENCES
[The birth of synthetic dyeing](https://www.open.edu/openlearn/history-the-arts/history/history-science-technology-and-medicine/history-science/the-birth-synthetic-dyeing)
[William Henry Perkin](https://en.m.wikipedia.org/wiki/William_Henry_Perkin)
[World's first synthetic dye](https://blog.scienceandindustrymuseum.org.uk/worlds-first-synthetic-dye/)
[Life history of William Henry Perkin](https://www.encyclopedia.com/people/history/historians-miscellaneous-biographies/william-henry-perkin)
[Dye](https://en.m.wikipedia.org/wiki/Dye)
[Electromagnetic spectrum](https://en.m.wikipedia.org/wiki/Electromagnetic_spectrum)
[Pigment](https://en.m.wikipedia.org/wiki/Pigment)
[How chemistry colours our world](https://www.elsevier.com/connect/how-chemistry-colors-our-world)
[Azo coupling, Diazotisation and Diazonium salts](https://en.m.wikipedia.org/wiki/Azo_coupling)
 )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
) )
)
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Congratulations @empressteemah!
Your post was mentioned in the Steem Hit Parade for newcomers in the following category:
I also upvoted your post to increase its reward
If you like my work to promote newcomers and give them more visibility on the Steem blockchain, consider to vote for my witness!