Solid-liquid extraction
The first is the most used and it is about this writing of the extraction with the Soxhlet team. As an example, all the active ingredient acquisitions of the plant tissues can be mentioned. The second has uses especially in analytical chemistry when the product is extracted from a reaction carried out in liquid phase with a specific solvent to separate one or some of the components. Finally an example of the third, gas - liquid, which is ordinarily called gas scrubbing, is the bubbling by a liquid phase of a gas that is to be washed or purified.
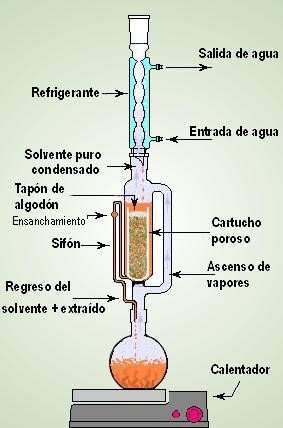
What the Soxhlet extractor does is to make a lot of extractions automatically, with the same solvent that evaporates and condenses, always reaching the material purely.
The Soxhlet extraction is based on the following stages: 1) placement of the solvent in a balloon. 2) boiling the solvent that evaporates to a reflux condenser. 3) the condensate falls on a container containing a porous cartridge with the sample inside. 4) Ascent of the level of the solvent covering the cartridge to a point where the reflux that returns the solvent with the material extracted to the balloon occurs. 5) This process is repeated as many times as necessary for the sample to be exhausted. The extracted is concentrated in the solvent ball.
Once the equipment is armed, open the water coolant, loaded the cartridge with sample and introduced the solvent, it only remains to turn on the heater and start the operation. When the temperature reaches the boiling point of the solvent, it starts to evaporate and, after the walls of the equipment are heated, it begins to condense in the refrigerant and to fall in drops on the cartridge. The first operation is totally atypical and should not be counted in the count that is done to regulate the speed of extraction as the rules usually ask for. As the condensate is falling on the cartridge, this
begins to run down the bottom of the same filling the extraction vessel to Siphoning that reaches the level of the descent of the siphon and rechupa, with all the dissolved material, towards the bottom balloon. The top of the siphon is above the cartridge to ensure that every time the material to be extracted is imbibed in the solvent.
Once the system is in regime the siphonings occur at regular intervals. The common times of siphoning are between 5 and 20 minutes, depending on the power of the heater, the sun-vente, the external temperature, etc.
The number of siphonings are stipulated in the standard that is used, but there are opportunities in which you work in systems over which information is not available. For that it is interesting to know with some approximation the general behavior of the extraction
Once the extraction operation has been terminated, it is convenient to wait a certain time for the system to cool until it is easy to manipulate it. Then do not forget to close the cooling water to avoid unnecessary consumption. Then the equipment is disassembled and the cartridge that is saturated with solvent is removed and placed in an aerated place or in the hood to dry the sample. Removing the sample from the wet cartridge can cause deterioration. If necessary, the extractor should be rinsed so that it is ready for the next time. And with this the extraction operation is terminated.