Hello, Steemians! I Hope everyone is fine. Today, I will be discussing the ultimate fate of our universe.
Introduction
Let me start with the Sanskrit verse to give stereotypical validity to this article. :P
जातस्य हि ध्रुवो मृत्युर्ध्रुवं जन्म मृतस्य च।
This is the famous verse from Hindu scripture Shrimad Bhagavad Geeta. I ain't completing verse as only one line is relevant here. It means death is certain of which is born. Don't take death literally so that we can generalize this for all entities. Then, the universe has existence now, it must cease to exist at some point. Then what would happen at that point? what is the ultimate fate of this universe? well, this article is all about it.
Let's come to everyday life.
Heat flows from hotter object to colder object, dye mixes uniformly in water, air particles at high-pressure flow towards lower pressure region, an object tries to be in minimum level in earth etc. unless we don't interfere with the system. It seems that nature is trying to distribute energy equally to all the systems. From this examples, we know that the energy in chunk form tries to be distributed among system and surrounding. This phenomenon is called entropy.
Entropy
Now, to the definition:
"Entropy is the measure of the degree of randomness".
Entropy is a physical quantity as it measures the randomness of the system. Randomness means a possibility of different states of particles in the system. Molecules in ice have a lesser possibility of states but in water, the molecules are in random vibration and with more possibility of states. So, we can say the entropy of water(liquid) is more than that of ice(solid). In cold body, the molecular vibration and possibility is less than that in the hot body. So, the entropy of hot body is more than that of the cold body.
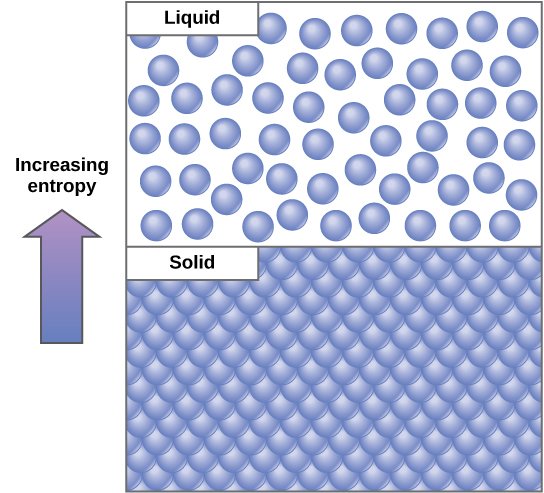
we can say that nature acts in a such a way that the system from lower entropy advances to higher entropy. From this observation in real life, we can gain something for the paper. If the entropy of system increases, the process is spontaneous. This happens in nature without external work. Tf the entropy of the system increases, it is a non-spontaneous process. It needs some external work to occur.
Example
At standard atmosphere, the ice changes to water without doing anything. So, it is a spontaneous process and entropy of the system(ice) increases in this process.
The water doesn't change to ice automatically. We need to do external work to make this happen. So, it is a non-spontaneous process and entropy of the system(water) decreases in this process.
Now to some physics and mathematics;
Second Law of Thermodynamics
The Second Law of Thermodynamics states that:
The state of entropy of the entire universe, as an isolated system, will always increase over time.
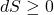
Thermodynamically,

dQ = small heat change in the system
dS = small change in entropy due to process
T = Absolute temperature of the system.
Loss of extractable energy
Let there be two reservoirs A and B with temperatures T1 and T2 (T1 > T2) respectively and let T be the temperature of the atmosphere.
If we extract Q amount of heat from reservoir A. The maximum amount of work that can be extracted from two temperature is by operating it under Carnot cycle. It is given by;
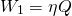
 is efficiency.
is efficiency.

So, 
Now Q amount of heat is transferred from reservoir A to reservoir B . This is a spontaneous process(T1>T2). Then, the work extractable from reservoir B is

Initially, we could have extracted W1 amount of work. But, the amount of work that can be extracted decreased to W2 due to spontaneous process.
This is called the loss of extractable energy or unavailable energy.





It is for the irreversible heat transfer process.
Conclusion
Spontaneous processes are happening all the time in nature. During this spontaneous process, the amount of extractable energy is decreasing. Consequently, the entropy of the universe is increasing. Due to this, there will be a time in future when there won't be extractable energy. In that condition, all the particles in the universe will be in thermal equilibrium i.e. temperature of all the particles in the universe will be same. This condition is called the heat death of the universe.
References
Dincer, I., & Cengel, Y. A. (2001). Energy, entropy and exergy concepts and their roles in thermal engineering. Entropy, 3(3), 116-149.
Kesidou, S., & Duit, R. (1993). Students' conceptions of the second law of thermodynamics—an interpretive study. Journal of Research in Science Teaching, 30(1), 85-106.
Zoline, P. (1967). The Heat Death of the Universe. Busy About the Tree of Life.
The song of God: Bhagavad-Gita. New American Library, 1951.
Good post. This reminds me of my post: The False Vacuum - One Of The Scariest Concepts In Physics
I think the heat death is another one of those scary concepts.
Maybe by the time it occurs, the species that has evolved from humans will have figured out how to hop over to a younger and hotter universe.
Congratulations @thescienceguy! You have received a personal award!
Click on the badge to view your Board of Honor.
Congratulations @thescienceguy! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!