 Refrigerator.
(Author: Katty Jamez]: Flickr
Refrigerator.
(Author: Katty Jamez]: Flickr Introduction: The laws of thermodynamics and the lawbreakers.
Since my sophomore year in the University, I have been fascinated by the interesting science of thermodynamics. We were taught the classical aspect of thermodynamics which focuses on the fundamental laws of thermodynamics on a macroscopic perspective.
If two bodies have the same temperature as a third body, then those two bodies are in thermal equilibrium with each other. This law of thermodynamics is actually the third law in the series to emanate, but because of the negligence of the first two laws to recognize this important definition of thermal equilibrium and its fundamental implication on temperature measurement of naturally occurring processes, it had to be called the zeroth law of thermodynamics. The third body, as the basis of temperature measurement between two other bodies, can be taken as a thermometer.
The first law of thermodynamics is based on the popular law of energy conservation which confirms that the total amount of energy in the world is constant and only transforms from one state to another. Energy cannot be created, neither can it be destroyed. For instance, literally, the transformation of solar energy to electrical energy with the use of photovoltaic cells neither implies the destruction of solar energy nor the creation of electrical energy. It is simply the conversion of one form of energy to another. Since not all of the solar energy received by the photovoltaic cells is actually converted to electrical energy, the rest is given off as heat (another form of energy).
All processes must satisfy the first law of thermodynamics to occur in nature. However, processes in nature also occur in a particular direction. This brings us to the second law of thermodynamics and the lawbreakers. This is best described by the popular cup of hot coffee placed in a cooler room experiment. In fact, the assertion of this simple experiment cuts across the zeroth law (thermal equilibrium), first law(energy conservation) and the second law (energy direction) of thermodynamics.
 Cup of hot coffee in a room.
(License: Public Domain]: Pixabay
Cup of hot coffee in a room.
(License: Public Domain]: Pixabay When you make a cup of hot coffee and have to leave it in a room to probably go and attend to the noisy disturbances of your neighbour. You would discover, if you have a really stubborn neighbour, that you would be greatly delayed. And by the time you return, your once hot cup of coffee would have become cool.
The simple explanation is this; while you were away trying to convince your noisy neighbour to stop disturbing the neighbourhood with his crazy sound system, the hot cup of coffee was having a thermodynamic interaction with the cooler air in the room.
What happens here is that heat is transferred from the higher temperature body (the coffee) to the lower temperature body (the room). This transfer continues until both bodies arrive at the same temperature. This occurs every time a hot body is brought close to a cooler body. The temperature exchange can never go from the cool body to the hotter body by itself. Another proof that nature is always generous. This decreasing direction in the quality of energy is the assertion upon which the second law of thermodynamics is based. The second law places a restriction on the direction of processes, which is something the first law did not put into consideration.
 A law breaker
(License: Public Domain]: Pixabay
A law breaker
(License: Public Domain]: Pixabay However, wherever you find laws, you are bound to find some lawbreakers. These lawbreakers, back in school, where usually known as the cool guys. The guys the girls want to be with and the other guys want to be like. I doubt they dress anything close to what is shown in this picture (to your right), but that's the best I could find :p
In thermodynamics, these lawbreakers are devices that reverse the direction of heat transfer from the low-temperature body to a high temperature one, 'breaking' the second law of thermodynamics it seems!
These devices are known as refrigerators and air conditioners. Well, could it be a coincidence that these guys are literally designed specifically for cooling purposes? Maybe not. Why have they not been brought to face the justice of the law? Okay, this is actually because they break the law by operating on a cycle that actually obeys the law. This cycle is known as the Refrigeration Cycle.
I know! I know! It’s almost as if I’m no longer making some sense. Don’t worry, an explanation of the refrigeration cycle would help us to appreciate better how the whole thing works.
The Refrigeration Cycle
In the refrigeration cycle, a refrigerant is made to undergo a cyclic movement between two heat exchange devices; the low-pressure, low-temperature evaporator and the high-pressure, high-temperature condenser. The refrigerant absorbs heat to become vapour in the evaporator and condenses to become liquid at the condenser, releasing heat. The compressor and the expansion valve are the two other devices that make up the Four major components of the refrigeration cycle.
There are many different forms of the refrigeration cycle. One of such is the Reversed Carnot Cycle, which, as the name implies, is a counter clock-wise operating Carnot Cycle. This is simply possible because of the reversible nature of the Carnot Cycle. The Carnot Cycle is not related to our discussion today but you can decide to read more about it here.
The major impracticality associated with this form of refrigeration cycle is the fact it consists of a turbine which would be subjected to expansion and also, the compressor has to deal with two phases of the refrigerant at the same time. There are compressors that can get damaged from pumping liquid.
The Vapour-Compression Cycle is a more practical cycle. It simply resolves the issues associated with the reversed Carnot cycle by ensuring that saturated vapour is what gets to the compressor and also eliminating the need for a turbine, by replacing it with a constricting device such as an expansion valve. This particular cycle is what is commonly used in our refrigerators and air-conditioners and what is going to form the basis of our discussion of the refrigeration cycle.
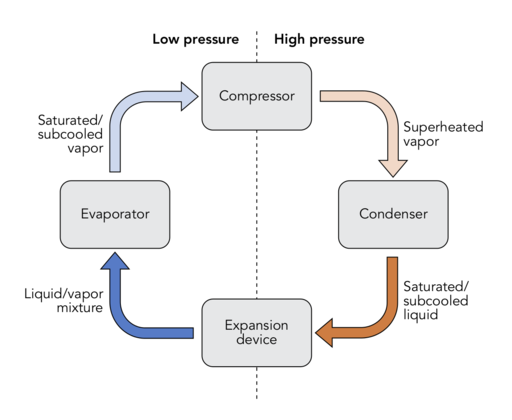 Vapour-compression refrigeration cycle
(License: CC BY-SA 4.0, Author: WGisol]: Wikicommons
Vapour-compression refrigeration cycle
(License: CC BY-SA 4.0, Author: WGisol]: Wikicommons The vapour-compression cycle comprises of 4 major components as can be seen in the image to your right; the compressor, the condenser, the expansion valve, and the evaporator which are connected by a refrigeration copper tube. The refrigerant goes round the cycle through the copper tube as it goes from one component to the other, doing work and transferring heat.
The Compressor
The compressor has two operating lines – a suction line which connects the compressor to the evaporator and a discharge line which connects the same device to the condenser. When the refrigerant arrives the compressor as a low-pressure, low-temperature gas (saturated vapour), here’s what happens; this saturated vapour is compressed to become a high-pressure, high-temperature superheated gas which is then transferred to the condenser through the discharge line. The pressure increase achieved in the compressor is what makes the refrigerant to repeatedly flow through the cycle.
The Condenser
The condenser is a heat exchanger. This is where the refrigerant, which comes in as a superheated vapour, undergoes condensation and converts to a saturated liquid, with heat given off to the cooler surrounding. Does this remind you of the hot cup of coffee placed in a room? I guess it does. It is in the same wise that the high-temperature refrigerant increases the temperature of its surrounding air until it in turn cools off into a liquid. This is why you would notice that the air behind the refrigerator is usually warm, the coils you see behind your fridge are the condenser coils.
The Expansion valve
This is the next destination of the now saturated-liquid refrigerant after leaving the condenser. As earlier mentioned, the expansion valve is a constricting device. What it does is to restrict the flow of the incoming liquid such that its pressure is lowered before getting to the evaporator.
The Evaporator
This is the second heat exchange device. It is often located where the actual cooling/freezing is to take place. The low-pressure, low-temperature refrigerant, upon getting to the evaporator, once again obeys the second law of thermodynamics; it absorbs heat from the warm air surrounding till the area becomes cool (your room or your fridge) while the refrigerant evaporates into a saturated vapour. This vapour is then transferred back to the compressor through the suction line, and the cycle is repeated in that manner.
Simple Uh? Right.
What about the fan and the liquid droplets?
I used to think that the fan blades located in the outdoor unit of the residential split air conditioner were singularly responsible for the cooling of our rooms. Don’t blame me. I was just a child.

) Outdoor AC units
(License: Public Domain]: Pixabay
Outdoor AC units
(License: Public Domain]: Pixabay The fan is actually responsible for the expulsion into the environment of the hot air given off from the condenser as the superheated vapour is converted to saturated liquid. This explains why the fan blades blow hot air to the outside environment.
The droplets of liquid formed during the evaporation of air in the indoor component are discharged out through the condensation drainage tube which we also see on the outside.
Let's talk about the refrigerant
The refrigerant is the life blood of the refrigeration cycle. It is the working fluid. Tirelessly travelling round the cycle, taking different forms at different stages and doing all the work expected of it to do. It wouldn’t be fair if we go by without giving it an honourable mention.
Refrigerants come in different form and types depending on purpose of application. In the air-conditioning of aircrafts, air is the preferred refrigerant; water is preferred for applications above freezing point; carbon dioxide, Ammonia and Chlorofluorocarbons (CFCs) can also serve as refrigerants for different applications.
Ammonia is the top choice refrigerant in the industrial sector because it has low energy cost, high heat transfer coefficient, can easily be detected in the event of a leak, and a couple of other favourable thermodynamic properties. It is however not suitable for domestic use due to its high toxicity which could threaten human health in the evnt of a leak.
This is why CFCs are preferred for residential and light-commercial usage. They also come with low cost and are highly versatile as they are also used in aerosols and as electronic cleaning solvents. According to Yunus Cengel and Michael Boles;
CFCs such as R-11, R-12, R-22, R-134a, and R-502 account for over 90 percent of the market in the United States.
R-12 (trade name – Freon) is the common choice for domestic refrigerators and air conditioners while R-22 is used mainly in air-conditioning of commercial buildings and large industrial refrigeration systems.
Conclusion: Any difference between the refrigerators and air conditioners?
It is pertinent to reiterate that the energy cannot neither be created nor destroyed. As such, an air conditioning system (ACs and refrigerators) itself does not create heat, it only transfers heat from an area where it is not wanted to an area where it makes no significant difference (sink).
We have already found out that these two lawbreakers are best of friends. Probably brothers. The major difference is in purpose. You wouldn’t buy a refrigerator to condition the air in your room. Would you?
If you answer yes to that, then I give up.
Refrigerators are used to regulate temperature for the purpose of cooling and freezing of food and other products. Air conditioners, on the hand, are used to regulate temperature and humidity of air in a room.
So that’s about all I have on the cool guys of thermodynamics. I hope you enjoyed the read and learned a few things.
REFERENCES
Basic Refrigeration Cycle. Retrieved on June 22nd, 2018
Heat pump and refrigeration cycle. Retrieved on June 23rd, 2018
Difference Between AC and Refrigerator. Retrieved on June 23rd, 2018
Cengel Y.A., Boles M. A. (2005). Thermodynamics: An Engineering Approach, 5th edition. New York, USA: McGraw-Hill Higher Education.
Hi @writeit!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Nice little post but it's wrong to say that Refrigerators and Air Conditioners break the second law of thermodynamics. According to the second law of thermodynamics, the heat never flows from a cold body to a hot body spontaneously. Refrigerators and Air Conditioners do transfer heat from a lower temperature to higher temperature but they require external work. The entropy change is also positive in these machines, so they don't violate the second law :)
Thanks for the contribution.
I expounded on that within the post. Since the second law of Thermodynamics asserts that heat never flows from lower temperature to higher temperature and there are devices that can do that, it may look to a common man that those devices are breaking the law.
But i explained why what one may think at first isn't really the case and how, in fact, refrigerators and air conditioners obey the second law to function.
Thanks for stopping by. I appreciate.