Hello hivers, my esteemed readers, erudite colleagues and my awesome students of chemistry. It’s been couple of weeks I last posted here on hive, I’m glad to be back. So, on my blog today, I will be sharing with you some of the uses of halogens; both the good and the bad side. But, before I go further, I need to remind you what halogens are. I’ve come to observe that many people assume or think that halogens are just about the brightly-lit lamp popularly known as “The Halogen Lamp” but alas! Halogens are much more than that.

The name, halogens, is coined from the greek word; “hals” and “gen” which means salt producer. They are called salt producers because when they react with metals, they form different types of salts including our common table salt. Halogens is a collective name used for the non-metallic elements in group 7 of the periodic table. It was Jons Berzellius; a Swedish scientist that first used the word “halogen” to show that their elements, chlorine, bromine and iodine were found in sea water. Other members of the group include fluorine, astatine and tennessine.
There are numerous uses of halogens, in which the entirety can’t be explained all in this post. Some uses are found in the production of photochromic glasses, cloud seeding, disinfectants, photography, lighting, warfare and in medicine. But, I will like to explain some its rare uses in photography and in warfare and then the impacts on the environment.
BLACK AND WHITE PHOTOGRAPHY
The traditional black and white photography is made from the reactions of ionic halides. But, what are ionic halides? I’m sure you would ask me this question.
Ionic halides are made when halogens react with metals. Most metals react when the hot or burning metal is plunged into an atmosphere of the halogen. Ionic halides show the properties similar to that of compounds with a giant ionic structure. They have high melting and boiling points, do not conduct electricity as solids, often dissolve in water and the solution will conduct electricity. However, some metals can form ionic halides with covalent character. These have much lower melting points than those of the other ionic halides, and often sublime instead of melting. Aqueous halide ions undergo both displacement and precipitation reactions. Most halides are soluble like I’ve said above, but silver halides are an exception because they do not dissolve in water. Therefore, silver halides can be precipitated by mixing together aqueous solutions of silver nitrate and the appropriate halides:
Ag+(aq) + X-(aq) → AgX(s)
Where X is either chlorine, bromine or Iodine. These precipitation reactions are very useful in qualitative analysis. It is observed, from the reaction, that the white precipitate of silver chloride has a grey tinge. This is because silver halides decompose slowly when left in sunlight, The grey tinge is metallic silver. It is this decomposition that is used in black and white photography.
Silver halides are sensitive to light. For example, in the precipitation reaction shown in the equation above, the silver chloride formed turns grey-violet in the presence of light. This is because of the decomposition of silver chloride to give silver and chlorine:
2AgCl(s) → 2Ag(s) + Cl2(g)
The longer the exposure to light, the greater is the amount of silver produced. Traditional black and white photography works on a similar principle. The film consists of a very thin layer of gelatine and silver bromide crystals deposited on clear plastic. As light shines on the film, an image is captured by the silver bromide crystals. This image is developed by immersing the film in a developer that reacts only with those silver bromide crystals that have been exposed to the light. The developer reduces the silver bromide to metallic silver.

Next, the film is placed in a fixer, where the silver bromide crystals not exposed to light, and hence unaffected by the developer, are removed from the film. This leaves the film as a negative with black areas of silver where the film was exposed to light. The negative is then converted into a positive picture on photographic paper. Light is shone through the negative onto the photographic paper, which is coated with silver bromide. The action of light on the silver bromide produces an image that again has to be developed and fixed to give a permanent photograph.
BIOLOGICAL WARFARE – DIOXINS
Dioxins are present naturally in minute quantities and form whenever wood and certain other substances burn. However, the chemical industry has added a great deal more to the environment, much of it before the danger posed by this group of chemicals was recognized.
For seven years during the Vietnam war in the 1960s, US aircraft sprayed the jungle with a mixture of herbicides known as Agent Orange. It is regarded as one of the most disputed weapons the US army used in the Vietnam War. It consists of a mixture of 2, 4-D (2,4-dichlorophenoxyacetic acid) and 2, 4, 5-T (2,4,5-Trichlorophenoxyacetic acid). They are man-made chemicals and named Agent orange after the container in which they were stored. In high concentration, the chemicals have the power to make plants outgrow themselves and start shedding off their leaves. All this was done by the Americans in order to remove the jungle cover the viet were using as an hideout in the war. The plan was to destroy the foliage under which the Viet Cong could hide. In all, some 50 000 tonnes of Agent Orange were used. However, Agent Orange was contaminated with dioxins, in concentrations of about 2 parts per million. So, about 100 kg of dioxins entered the Vietnamese jungles. The subsequent births of babies with abnormalities provided the very first indication that dioxins cause genetic defects. Also much in evidence was a terrible skin complaint called chloracne, which was caused by exposure to dioxins.
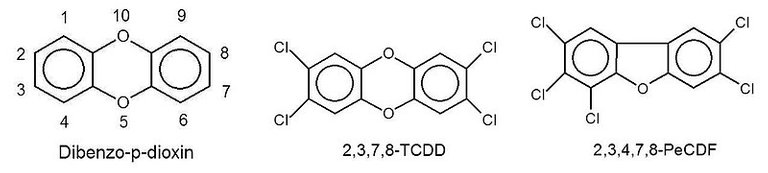
In 1976, an accident at a chemical plant in Seveso, Italy released dioxins into the air. There was an immediate outbreak of chloracne and 600 people were evacuated from the area. This brought dioxins to public attention. Some dioxins are harmless, but many are not. One of the most deadly is TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin). Bleaching wood pulp with chlorine to produce paper products, such as newsprint and disposable babies’ nappies, has been known to produce minute quantities of dioxins, including TCDD. This has led to a phasing out of chlorine as a bleaching agent. Chlorine dioxide provides a safer alternative.
Should we continue to be alarmed about dioxins? Now everyone is aware of the dangers of TCDD and the other deadly dioxins, the means of their production are being reduced, which is bringing levels down significantly. For example, dioxins are produced by leaded petrol, so the switch to unleaded petrol has led to a notable reduction in air pollution by dioxins. Attention is now focusing on improving the incineration of waste – another source of dioxins in the environment.
THE IMPACTS ON THE ENVIRONMENT
One of the applications of chlorine, an halogen, as an element is to bleach the wood pulp used to make paper products and textiles. Highly toxic dioxins are by-products of this process, most of which are carried away in the waste-water. So, there is growing concern that this process is helping to increase dioxin levels in the environment. There is also evidence that dioxins do enter the paper products themselves – another cause for concern. This application of chlorine is being gradually reduced and could eventually be phased out altogether. There is also a move to find an alternative to chlorine for destroying harmful microbes in the water supply. But any alternative must be proved to be as effective as chlorine before the switch can be made. Ozone is a possibility, but it breaks down rapidly and loses its disinfectant properties. Therefore, it offers no protection against reinfection of the water.

Almost 30 per cent of all chlorine produced is used to make the monomer chloroethene (CH2=CHCl), from which PVC – the world’s most versatile plastic – is manufactured. Demand for PVC is likely to continue to grow. There are some fears over the safety of the plasticisers in this polymer, particularly in its use as food wrapping. Another issue is how it should be disposed of. Incineration can produce minute quantities of dioxins. However, there would seem to be no case for banning this chlorine compound, since virtually all of its uses are safe and its disposal could be made as safe.
Solvents are among the organic products made using chlorine. The production is being phased out of those known to cause depletion of the ozone layer in the upper atmosphere. All chlorinated organic solvents are bound to come under increasing scrutiny. While the dangers of chlorine and its compounds must never be denied, blanket condemnation of them is not realistic. Each of the numerous chlorine-based compounds now produced deserves to be assessed individually according to its advantageous properties, its toxicity and its environmental effects. Many of the most effective medicines and pesticides are chlorine-based compounds, and without them, disease would be more widespread and crop production would fall.
Thank you for reading.
@tipu curate
Upvoted 👌 (Mana: 6/12)
Thank you very much for your long term contribution to our label with your educational publications, I hope this will last a long time!
You are welcome. I'm glad to be a part of this community. Thank you all for the unwavering support, @carloserp-2000.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app
and including @stemsocial as a beneficiary, which give you stronger support.
Congratulations @empressteemah! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @hivebuzz:
Support the HiveBuzz project. Vote for our proposal!