The molecules
Version française : Steemit | Busy
Here we go, it was necessary that we talk about this! After all, this is that composed everything that we know (water, wood, air, …), a bit like the cement, which is omnipresent in building.
Wait, you told me at the beginning that it was atoms which composed all this matter, so what it the difference?
 Let’s see that closer.
Let’s see that closer.
Atoms, molecules? Actually, a molecule is an assemblage of atoms! Like a Lego® building, where atoms would be the single piece and the molecule the final (or not) assemblage.
OK! So, let’s build some molecules! First you to know with what you will play, there is the list:

Source : http://supered2k.eklablog.com/tableau-periodique-des-elements-pdf-tpe-p1100216
TADAM! We called that the periodic table, each case that you see is an atom type. This table is exhaustive… until proven otherwise (the favorite sentence of scientists). Most of the atoms are metals or gas (in orange) expect these in red and white. So, these atoms are linked with other atoms with bonds.
Game rules are simple, each atom must have a certain number of bonds with other atoms varying from 1 to 4 bonds. This number is fixed for each atom, for instance, the [H] can’t have more than one bond, therefore, the [Si] must always have 4 bonds.
That is a water molecule (super well drawn):
 Hence the H2O
Hence the H2O
No panic, today we will use just a little part of the table! Eh yes, because we will deal with molecule from the living! Welcome the organic chemistry!
Living organisms use atoms that are in abundance, which means that most of molecules have a poor diversity of atoms. Look:
[C], [H] and [O] are the three elements that are in abundance, here are their characteristics:
- [H] for Hydrogen, it has only one bond possible
- [O] for Oxygen, this one must have 2 bonds
- [C] for Carbon, and this one must have 4 bonds
By the way, what defines a molecule from the living world?
Nothing complicated, it just must have C – H bonds!
Although there is a poor diversity of atoms there is a huge diversity of assemblage!
But me, when I heard molecule I see this:
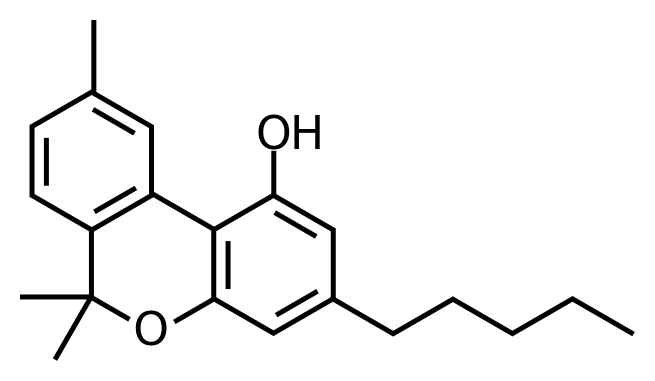
I don’t understand what is these bars without letters, where is [C] by the way?
 I love this molecule!
I love this molecule!
So, this is a simplification; indeed, [C] and [H] are in abundance, so we don’t need to specify them each time. In the molecule above, each angle (including edge) is [C]. So, we can enumerate them: this molecule have 21C, you get it?
But where are the [H]? For the number of [H], we go back to the [C] characteristic, this one must have 4 bonds with other atoms. So, we must fill each Carbon with [H] to have 4 bonds on each carbon. Let’s enumerate the [H]! I get 26 with the H on the [O], you find them? (If you have 33 or 37, look below!)
Now let’s see the double bars, these are double bonds, it means that [C] is linked with another carbon with two bonds (C=C), so, these have fewer bonds with [H]. It also exists triple bonds.
A molecule has a structure like this one that you show me above, and a molecular formula, which is the total of atoms. In our case, the molecular formula is C21H2602.
Now I’m sure that you can give me the name of the molecule and the name of the plant that comes from.
 I wait for your answer!
I wait for your answer!
Let’s continue! Molecules have functional groups, which is a little part of the molecule, the simplest are the functional group alcohol represented by a –OH, you have one in your molecule by the way.
Oh, cool alcohol!

When I talk about alcohol, I don’t talk about vodka or gin, actually, all alcohol that we drink and give us “superpower” contain the same molecule: the ethanol.
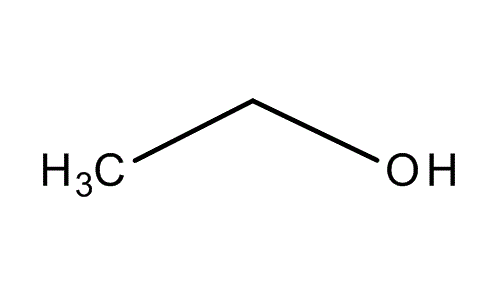 I’m sure that you can give me the molecular formula.
I’m sure that you can give me the molecular formula.
The list or functional group is big, you can find it on Wikipedia, but it’s not useful for the moment.
Organics molecules are classified in different groups and sets. In short, there is a big classification, roughly speaking, here are few classes:
- Proteins
- Lipids
- Glucids
- Nucleotides
- Amino acid
In the next chronicle on molecules, I will talk about lipids, I promised you!
When? When I talked about cell membrane, indeed, the cell membrane is composed of lipids!
Référence :
https://fr.wikipedia.org/wiki/Boisson_alcoolis%C3%A9e
https://fr.wikipedia.org/wiki/%C3%89thanol
https://fr.wikipedia.org/wiki/Tableau_p%C3%A9riodique_des_%C3%A9l%C3%A9ments
https://fr.wikipedia.org/wiki/Groupe_fonctionnel
https://fr.wikipedia.org/wiki/M%C3%A9tabolisme
What this Dr.Plantes want me?

#01_Where are we? What time is it?
#02_The bacteria
#03_The DNA
#04_The evolution
#05_The cell
#06_The fission
#07_DNA replication
#08_The reproduction