Browsing science news I came across THIS press release from UC Davis, with a title "Common Antiseptic Ingredients De-Energize Cells and Impair Hormone Response: Some Disinfectants Inhibit Cell Energy and Alter Reproduction." This sounds quite definitive. So I decided to find the source material (the research done by UC Davis scientists) and have a look for my self. After doing so I thought maybe some of you might also enjoy a deep dive into this piece of work.
So today we will be breaking down some work recently published in the journal Environmental Health Perspectives, an Open Access Publisher, titled "In Vitro Evaluation of Mitochondrial Function and Estrogen Signaling in Cell Lines Exposed to the Antiseptic Cetylpyridinium Chloride."

What Were The Authors Studying Here?
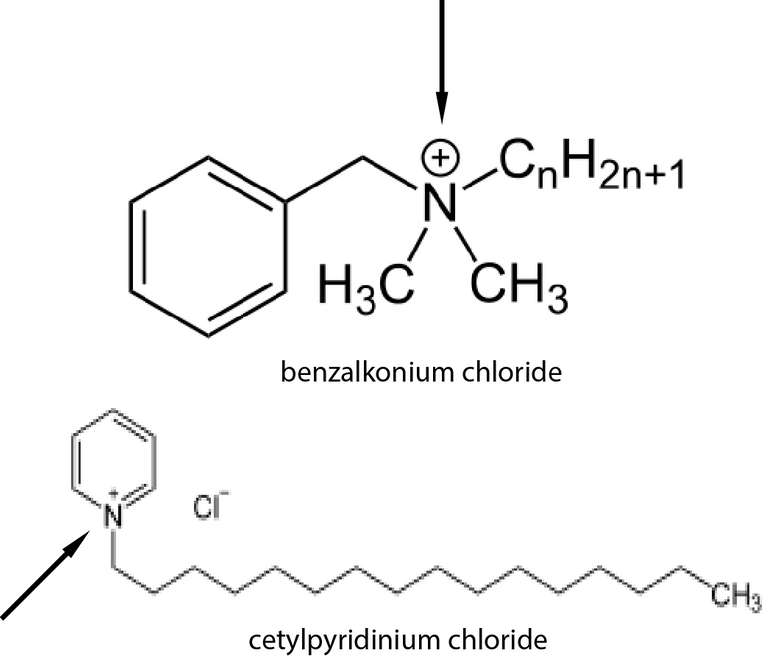
The authors were studying compounds known as quaternary ammonium salts (they looked at two of them specifically, one called cetylpyridinium chloride (CPC) and another called benzalkonium chloride (BAK). These compounds are called quaternary ammonium salts because they contain a quaternary ammonium (... you are probably shocked!), which I have identified for you with the arrows in the image to the right.
These compounds are used for their antimicrobial properties, where they work to disrupt the bacterial cell membrane making it porous and leaky. [3]. This is an advantageous property, and as a result these compounds have been very widely used as topical (on the surface) antiseptics (and as household disinfectants in cleaning products) since the 1930s.[4], [5]
They are currently used to preserve things that we all use every day including toothpaste, hand lotions, mouth washes, deodorants and eye drops.[6] However, as the authors discuss in the publication there have been some reports coming out in the literature indicating that a few of these quaternary ammonium compounds inhibit mitochondrial functioning.[7] These sorts of findings appear to have resulted in the US FDA removing the tag of "generally recognized as safe" for one of the two compounds studied below (CPC) in certain products, however it's use remains in a variety of others (cleaning products).
The Data from the Datta Et Al. Study (today's article)
In this study the authors were looking more into the effects that CPC and BAK have on mitochondrial functioning. They begin by reporting on two parameters that serve as biomarkers for mitochondrial functioning, O2 consumption and ATP synthesis.
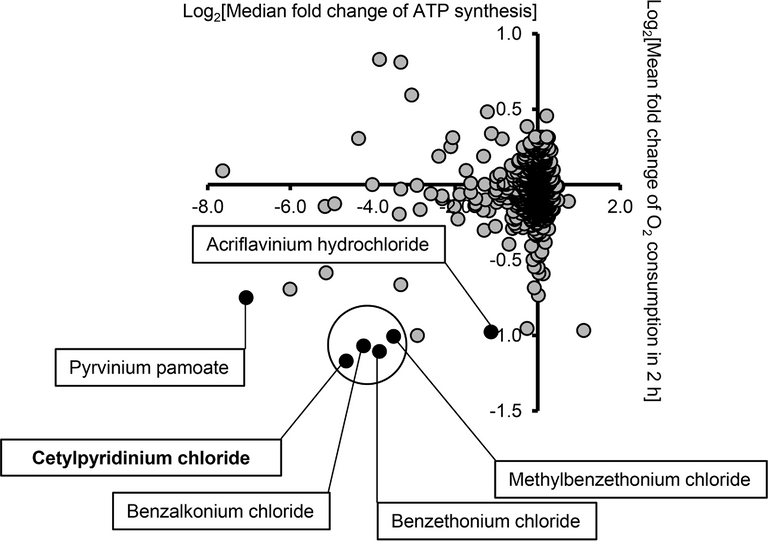
They screened a library (a fancy science way of saying a bunch) of commercial additives, disinfectants, drugs etc, against some cells and used a bioluminescent (these are techniques that allow us to monitor a cellular or in this case mitochondrial reactions through a secondary compound which emits light) technique to analyze these two mitochondrial activities.
As you can see in the image to the left, a few of the tested compounds fell in the lower left hand quadrant, this is where they observed both a reduced O2 consumption rate, as well as a reduced ATP production rate. They found that six of the compounds that fell in that space were quaternary ammonium salts, and the list of those included both CPC (cetylpyridinium chloride) and BAK (benzalkonium chloride).
Okay, lets not get ahead of ourselves. This data indicates that mitochondrial function was inhibited, but they were using relatively high concentrations of the compounds in the screen (10 uM) well beyond the concentration which would be achieved in our bodies from exposure (unless you were drinking them). Another thing to note is the authors report that there were a number of quaternary ammonium compounds that did not show any mitochondrial inhibition in the screen.
Biocheimstry Time (lets look at CPC's effects)
The authors next wanted to look at the effects that CPC had in a little bit more detail so they did some biochemical characterization of the mitochondrial oxygen consumption (See Figure Below, A) and ATP synthesis (See Figure Below, B), with respect to varying amounts of the compound (the datapoints were determined after cellular exposure for 24 hours).
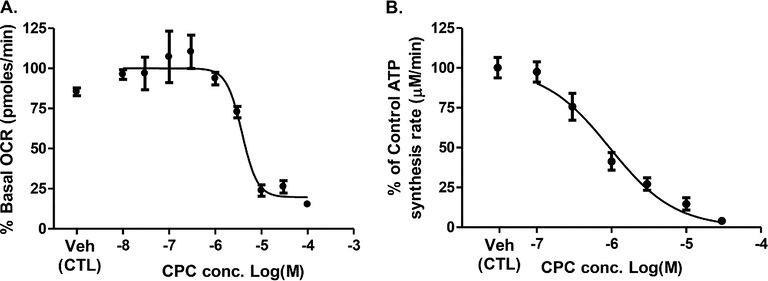
Now I understand that looking at a figure like this might be intimidating for many of you as you are likely not used to thinking about compounds in terms of concentrations, nor are you used to looking at data plotted in a log scale. So I will describe what the authors determined from this data, what they were interested in.
When you are studying a compound's effect on an enzymatic/cellular function you are often interested in determining how much of that compound is necessary to result in the change in function (whether it inhibits how the enzyme works, or activates the enzyme). To do this we use experiments like the authors did above, where they keep increasing the concentration of the compound until they see a change. In the case of this compound (CPC) they saw that the enzymes function decreased as the concentration of the compound increased.
They identified that the amount of compound necessary to reduce the mitochondrial oxygen consumption by 50% was 3.8 x 10-6 M. Or in-between the -6 and -5 on the X-Axis of (A). They also found that the amount necessary to reduce ATP synthesis by 50% was 0.9 x 10-6 M, or to the right side of the gap between the -7 and -6 on the X-Axis of (B). If you look at the plots these places are indeed where the lines are going down, and are halfway between the top points (when the mitochondria were still happy and active) and the bottom points (where the mitochondria were no longer happy and active).
The CPC Compound Inhibits A Mitochondrial Enzyme Called NADH-ubiquinone oxidoreductase
Based on the data shown above the authors thought that the CPC compound might be stopping the function of a micochondrial enzyme called NADH-ubiquinone oxidoreductase which is an enzyme involved in the electron transfer pathway necessary for mitochondrial metabolism. (It moves electrons from an energy storage molecule NADH to a different molecule called Coenzyme Q10].
They wanted to figure out whether this was true, so they again looked at Oxygen usage rates, only this time they were prepared to supplement with a molecule called succinate that entered the metabolic pathway at the next step after NADH-ubiquinone oxidoreductase. If the addition of the succinate restored the oxygen consumption when the CPC was around, then they were correct that the CPC was acting to stop the activities of NADH-ubiquinone oxidoreductase.
This is PRECISELY what they observed. Oxygen consumption slowed when the CPC was added, but upon spiking in some succinate the oxygen consumption went back up. The CPC was indeed inhibiting the functioning of the metabolically essential NADH-ubiquinone oxidoreductase.
Some Conclusions
The authors studied the effect of some commonly used antimicrobial compounds on mitochondrial functioning. They found that a some but not all of these quaternary ammonium compounds were able to negatively impact that mitochondrial function.
This data is interesting because it shows that there is a specific structural component to why these compounds are able to inhibit those mitochondrial functions (some compounds can and some can't). It is important going forward now, to better identify what it is about the compounds that are not inhibitory to mitochondria, this could potentially lead to the development of increasingly safer and equally effective topical antibiotics.
They conclude with the following statements:
In summary, our findings suggest that the QUATS CPC and BAK, which are used as disinfectants in consumer products, inhibit mitochondrial complex 1 and show antiestrogenic activity in vitro at low (micromolar) concentrations that may be physiologically relevant.
Meaning that the concentrations they tested may be achievable from normal exposure, but that is not known.
Overall, our findings strongly support the need for further investigation of the underlying mechanisms and potential consequences of chronic exposure to CPC and BAK in consumer products.
Indeed, as they have shown a convincing mitochondrial response, and even identified an enzyme which the compounds are likely inhibiting (NADH-ubiquinone oxidoreductase), further studies are clearly necessary to better understand both the risks associated with the use of these compounds in our household cleaning products, as well as to potential development of more of the compounds which do NOT result in a negative effect in mitochondrial functioning.
My Thoughts
This is not something I would be overly concerned with, but it is interesting how compounds that we thought in the past were safe for human exposure, upon further examination are identified to have unforeseen consequences, however minor.
I would truly like to see companies make the smart choice to shift toward different compounds in this same class, that do not have these detrimental effects. However... I'm not gonna hold my breath for that to happen.
Sources
Text Sources
- https://www.ucdavis.edu/news/common-antiseptic-ingredients-de-energize-cells-and-impair-hormone-response
- https://ehp.niehs.nih.gov/ehp1404/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1797692/
- https://med.nyu.edu/pophealth/sites/default/files/pophealth/QACs%20Info%20for%20Physicians_18.pdf
- http://onlinelibrary.wiley.com/doi/10.1002/cmdc.201100404/abstract
- https://www.ncbi.nlm.nih.gov/pubmed/12164747
- http://aac.asm.org/content/57/6/2631
Image Sources
All Non Cited Images Are From Pixabay.com, Flickr.com, Pexels.com, or Wikipedia.com And Are Available For Reuse Under Creative Commons Licenses
Any Gifs Are From Giphy.com and Are Also Available for Use Under Creative Commons Licences
If you like this work, please consider giving me a follow: @justtryme90. I am here to help spread scientific knowledge and break down primary publications in such a way so as to cut through the jargon and provide you the main conclusions in short (well compared to the original articles at least!) and easy to read posts.
SteemSTEM
Secondly, please consider supporting the @steemstem project. SteemSTEM is a community driven project which seeks to promote well written/informative Science Technology Engineering and Mathematics postings on Steemit. The project not only curates STEM posts on the platform through both voting and resteeming, but also re-distributes curation rewards as STEEM Power, to members of Steemit's growing scientific/tech community.
To learn more about the project please join us on steemit.chat (https://steemit.chat/channel/steemSTEM), we are always looking for people who want to help in our quest to increase the quality of STEM (and health) posts on our growing platform, and would love to hear from you!
Hello, @justtryme90, this post has been selected for @buildawhale's Curation Digest! Congratulations and thank you for your work!
https://steemit.com/buildawhale/@buildawhale/buildawhale-curation-digest-hf1-09-05-2017
Thank you kindly @buildawhale. Most appreciated. :)
:)
My poor mitochondria! It is scary to read that normal exposure to cleaning products, which we handle on a daily basis, may lead to the concentration levels they studied. Oh! 😳
May is an important word though. I would get scared if we thought that they DO lead to the concentration levels that they studied (and even then, I would like to see some pharmacokinetics data to learn how LONG the compounds stick around in the body, if they get destroyed really fast then its probably not an issue either as the concentration will drop out of a harmful range before any damage can be done). A lot of things may happen, this is just evidence for a definite need of further study.
My poor mitochondria! It is scary to read that normal exposure to cleaning products, which we handle on a daily basis, may lead to the concentration levels they studied. Oh! 😳
😜
Now you're killing me
the third user to copy the same comment. I see insane here lol.. I think if you started to sell this comment, you will get great profit hehe
Good post and great analysis of a complex paper. Too bad the conclusions are a bit wishy washy.
You should know not to make too strong of a declaration with out the data to back it up man. If the authors made bold claims then people would flip out about these anticeptics being used in household products, potentially without reason for it. No point in making a shitstorm over nothing.
Plenty of time for pitchforks with more data to support the pitchforks. ;)
yeah i try to cut-out antibacterial agents, like in dish soap... it's hard because so many things are loaded with triclosan, etc
Yeah, the worst thing about triclosan is it doesn't do anything any better than regular soap. We are developing more antibiotic resistance for nothing.
Very informative... Keep it up
Thanks!
Thank you for sharing this important information
Thanks for reading @team101
that's really a great informative post. thank you so much for being part of the community.
let me add you to my following list
Thanks! I am glad you enjoyed the post.
you are more than welcome
I only follow people who I think they deserve and if you see the number of users in my following list, you will know this
and I really see you are one of the most users that deserves to be followed
I don't know if I deserve it or not, but I appreciate that you feel that way.
these words are the strongest proof that you deserve it because great people don't say that they are great while others see them great
keep steeming on, you have my support starting from today
good find, hopefully more can be developed again for health world, interesting post.. @justtrymen90
Honestly, first time to read this information and I like to get new information always. that's really what's called adding value to the community.
thank you so much @justtryme90
Thank you for the reinforcing kind words. :)
you are more than welcome @justtryme90 , that's the truth not just words
Even minor negative changes can still cause the occasional problems when used for human interaction and exposure. Thanks for the share!
Thanks for reading. :)
I didn't know these info about toothpaste before, I think you will be really helpful to me if I follow you
that deserves tons of upvotes
I'm glad you got something out of it :)
thank you so much, I'm more glad to find you here and be one of the first people to follow in this community
Very informative and great science, i take a long time to study in graphic, i repeatedly look and trying to understand this graphic, mitochondria will inactive at lower -4, is it ? thanks for sharing
The numbers refer to concentration on a log scale so -4 would be 100-999 uM.
Great science report.
Since the abundance of healthy mitochondria in our cells is essential for our health, it's best to avoid things that can inhibit them.
Any suggestions on which products are potentially the least or most harmful to our mitochondria?
Co Q 10 is a well recognized supplement that helps mitochondria to function (and helps to reverse some of the damage caused by statin drugs.)
Symptoms of unhealthy mitochondria are muscle pain and weakness, this also includes the heart muscle, due to reduced ATP formation by our mitochondria. ATP is the molecule our muscles use for energy.
Unfortunately I don't have a list of which products contain what compounds. Would have to research on a case by case basis I think.
Very good discovery and very useful thanks @justtryme90 for your post I will share to another friend ..
Thanks for the kindness. Glad you found it informative.
You're welcome ..
very good post, very informative, truth never read about them, thanks for sharing
Thanks for reading.
Oh man this mitochondria is going to be in trouble due to our daily habits. Now we cannot imagine our life without these cleaning products. We really have to do something for it.
Do more research and change to the other compounds of the same class that do not show this effect, if indeed the concentrations get high enough from common use. It's really not a difficult fix. :) I don't think there is anything to worry about really.
Now I feel much relaxed. Thanx for letting this know. For refreshment of your mood, have a look at some of my cool arts.
Great to read your articles ..... Toothpaste damaging our mitochondria?..... the adverse effect seem to be minnor and proportionately less compared to the amount antiseptics/toothpaste we use daily.. but moving to the safer side is always wise..
Indeed good points.
I've never looked at it like this. Some of these compound that we see as "safe" are not too safe. One of my friends once told me that toothpaste is not safe. He advocates for brushing with salt; But I really can't imagine it.
I honestly wouldn't worry about it. It's worth knowing but I tried to make a point at the end to stress that this is not something to be overly concerned about. Toothpaste is perfectly safe.
Perhaps we should make changes to the type of antiseptics we use, but even that is not definitively clear. What this research indicates is that there is clearly a need for more study on this. Not time for panic yet.
Maan really informative post <3 it looks like you put a big effort on it, keep sharing my friend <3
Thanks for reading man. Appreciate it.
my up vote you have. in the toothpaste are to many things which are not healthy for us. But still there are inside. Flour is one of them. Did you try to made natural toothpaste anytime?
I don't think toothpaste is unhealthy to use. We're not eating it. I also don't lose any sleep over fluorine.
Nope I do not, nor will I ever use natural toothpaste.
Thanks for reading the post!
Nice post...about science dvlpment
thank you
You're welcome
thanks for making this
Thanks for taking a look :)
Thank for share @justtryme90
You're welcome. Thanks for taking a read @steembike.
This post has received a 2.70 % upvote from @buildawhale thanks to: @trumpman. Send 0.100 or more SBD to @buildawhale with a post link in the memo field to bid on the next vote.
Steem WitnessTo support our curation initiative, please vote on my owner, @themarkymark, as a
I have followed you on Steemit, please be kind enough to follow me as well.
Okay :)
This is a first for me. I have seen people copy others posts before... but never a comment..
https://steemit.com/science/@justtryme90/cetylpyridinium-chloride-toothpaste-and-mitochondria-oh-my#@abigail-dantes/re-justtryme90-cetylpyridinium-chloride-toothpaste-and-mitochondria-oh-my-20170904t004337880z
hahahaha..that's really insane, I can't stop being shocked on this. why should somebody copy another comment? it's the first comment in the page of @abigail-dantes
hahahhahahaha