Dear Steemiacs,
this post will be part of a series covering the science behind Nobel Prize awards in Medicine/Physiology. Here, I will deal with some of the implications of the 2017 Nobel Prize for discovering the molecular basis of the animal circadian clock. The scientific explanations are kept simple, yet require some basic knowledge about animal physiology.
If you want to know how and why one of probably the only two legitimate diet strategies have been indirectly acknowledged by the highest science court, and how, with a simple modification of your diet, you might get measurable improvements in your health parameters, then read on!
But beware! Long post, for tl;dr scroll down to the summary.
Life has evolved in a perpetual cycle of dichotomous time intervals, alternating between day and night, light and dark. During evolution, the presence of sunlight signaled availability of solar energy to photosynthetic organisms (plants and algae nowadays), and easy visual orientation, relative warmth and readiness for physical activity to diurnally foraging animals. Nocturnal animals, on the other hand, have adapted the active phase of their daily cycle to low levels of illumination exploiting the strengths of other sensory organs. Common to all organisms is, therefore, their potential to anticipate and prepare for periodic environmental changes in order to assume smart, resources-sparing behavior patterns.

Displaying this symbolic and amazing painting by courtesy of its creator, @errymil, check out his art channel!
I got very excited when I read the Nobel Prize committee's announcement on their decision to dedicate this year's award to researchers who have discovered and dissected the molecular clock machinery that endows organisms with the above postulated ability to synchronize their physiology to the externally provided day/night rhythms.

The freshly ennobled gentlemen: Jeffrey C. Hall, Michael Rosbash, Michael W. Young. Source (Chinese University Of Hong Kong Handout/EPA)
The fact that all higher-order organisms have a molecular clock ticking and governing the output of their genomes proves that organisms which have not learned to adapt to the terrestrial light/dark cycle have been outcompeted or have become extinct during the decibillions years of evolution.
You might understand my excitement if I told you that one of the most recent diet hypes has just been acknowledged by the Nobel Prize committee! If you haven't heard about Intermittent Fasting (IF) yet, I highly recommend to check out Martin Berkhan's blog - probably the first personal trainer and biomedical scientist to promote this idea within the fitness community.
Wait, wait, wait, you say. What does Intermittent Fasting have to do with molecular clocks?
Let me explain this idea in three blog posts: In this first part I will cover the rationales and the evidence for effectivity of Intermittent Fasting (IF) obtained from studies in rodents. In the second part I will present some of the existing evidence for the benefits of IF in humans, with a focus on the exercising population. In the third part I am going to briefly share my experience with this diet.
Let's begin with some general basics, partly attributed to the freshly baked Nobel laureates' research.
The periodic behavior of an organism is termed circadian rhythm (from circa = around and dies = day). The circadian rhythm results from the action of circadian clocks within the cells of an organism. Although circadian clocks are entrained by environmental cues, they can cycle autonomously even when the environmental phase cues are withdrawn, e.g. when mice are held in constant darkness.
The master circadian regulator organ in mammals resides in the suprachiasmatic nucleus (SCN) of the hypothalamus which itself is a conglomerate of different “task force regions” and the most important hormone homeostasis centre in the brain. As its anatomic name implies, the SCN is situated above the optic nerve chiasma, the crossroads between nerve bundles coming from both of the eyes retinae.
The SCN receives signals from specialized neurons in the retina and is thus able to register periodic light condition changes. Some important output signals from the SCN flow into the pineal gland, promoting melatonin secretion and preparing the organism for sleep. In addition, the SCN modulates the responsiveness of the nucleus arcuatus (ArcN) to the adipose tissue derived hormone leptin. The ArcN, in response, regulates hormones that confer feeling of hunger and satiety.
The SCN's function is, furthermore, to provide pace for the circadian clocks of peripheral organs, such as liver, adipose tissue and pancreas. They, in turn, determine the cyclic behavior of metabolism pathways and respond to feeding patterns.
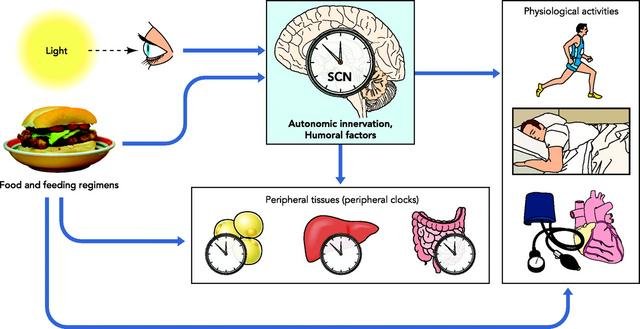
A schematic of the organisation of the human circadian rhythm system.
Sourced from Froy O, Circadian Rhythms, Aging, and Life Span in Mammals. Physiology 2011 Aug 12: 26(4);225-235
The great merit of the Nobel prize laureates' research was the discovery of the genetic and molecular basis for the ticking of the circadian clock. Their studies led to the following insights: The two phases of the daily cycle are governed by two opposing transcription factor complexes. Transcription factors are, simply put, proteins whose job it is to orchestrate a specific genetic program, similar to starting a software on your computer which then provides a certain output, like an anti-virus screen.
The dominant transcription factors during the inactive phase of the day (night time for humans, day time for rodents) are called CLOCK and BMAL1. They both cooperate and besides regulating genes that function in adapting the organism to rest and recovery, they switch on the production of their counterparts and their own repressors, the transcription factors PER and CRY, that slowly accumulate.
PER/CRY, when finally prevailing over and shutting down CLOCK/BMAL1, adjust the output of circadianly regulated genes to appropriate expression levels for the active phase of the day (day time for humans, night time for rodents). At the same time they enable the gradual build-up of CLOCK and BMAL1. These, in turn, promote the degradation of PER and CRY and the cycle repeats itself.
This regulatory loop was coined transcription-translation feedback loop (TTFL) and it is an elegant solution of nature to endow the genome with a binary-state-like cycling ability. Electroengineers might find analogies to electric circuits and I'd be glad if anyone would comment.
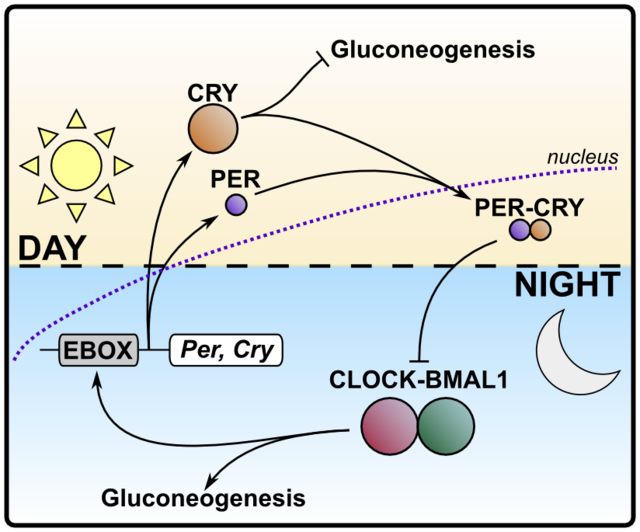
Simplified diagram of the interplay between the opposing molecular clock elements.
In humans, the CLOCK/BMAL1 pair is active during the night phase, while PER/CRY take over during the day. Exemplified is the regulation of gluconeogenesis. Bar-headed arrows signify inhibition. Source
Now that we have covered the very basics of the circadian clock, let's have a look at what studies on rodents have found out about how metabolism is regulated in a circadian fashion.
A remark, beforehand: Scientists often utilize mouse strains in which one or several genes have been deleted, allowing for studying the role that a gene usually plays by observing the phenotypes which result from the absence of it.
The studies that I am citing set out to investigate how the circadian rhythm regulates metabolism. They used normal (=wildtype) mice and mice that were deficient for BMAL1, PER or CRY genes to infer what effects disruption of the circadian molecular network will entail.
As an alternative to this artificial approach, they mimicked real-life human scenarios of jet-lag, shift work or persistent light conditions, by either keeping mice in periodically delayed and then pre-drawn light/dark phases or in constant light conditions.
A last primer is that mice usually thrive on a normal chow diet, containing 13% of energy as fat. When their diet is switched to a high-fat diet (61% of calories in fat), their circadian and feeding rhythms are “dampened” and they are prone to develop obesity and diabetes.
1. Corticosteroid-promoted glucose intolerance
Corticosterone regulates glucose homeostasis, including gluconeogenesis (the de novo synthesis of glucose) in the liver. Gluconeogenesis needs to be turned off during the waking/feeding phase of the circadian cycle since there is exogenously derived glucose entering the bloodstream. This is exactly what CRY does: It limits the ability of GR to turn on gluconeogenesis during the feeding phase, such as to maintain physiological, normoglycemic blood glucose levels.
When the CRY gene is deleted from mice, the animals experience a metabolic disturbance that resembles what is observed in patients treated with glucocorticoids for immunosuppression: steroid-induced diabetes. Although their behaviour is normal in terms of rhythmicity, CRY deficient mice display an impaired glucose tolerance when challenged with a bout of sugar, i.e. their blood glucose levels rise much higher and remain high for a longer time than in normal mice. CRY deficient mice fail to downregulate corticosterone levels during the inactive phase of the circadian cycle and therefore have constantly high levels of this hyperglycemic hormone.
Lastly, when these mice are chronically treated with synthetic glucocorticoids (dexamethasone), their fasting blood glucose goes through the roof and they show impaired glucose tolerance even to a light glucose challenge. Interestingly, CRY does not affect the levels of immunological target genes of GR.

Source
The implications of this study: circadian rhythm disruption can lead to unchecked action of the glucocorticoid axis leading to a diabetes type II-like condition with impaired glucose tolerance. The study might also have significance for the timing of medication in patients that need treatment with synthetic glucocorticoid drugs.
2. Insulin resistance
Most interestingly, BMAL1 deficient mice that are afflicted by arrhythmic behaviour and feeding patterns due to their lack of a functional circadian clock, seem to be constantly locked in this insulin-resistant and hyperglycemic phase. This correlates with a decreased activation of the AKT and mTOR pathways in the liver, a typical molecular indicator of insulin resistance.
The phenotype seems to remain subclinical and without consequences in terms of body-composition. However, when BMAL1 deficient mice are nutritionally challenged with a high-fat diet (HFD) for two months, they end up having double the fat mass of their control wildtype conspecifics.
The authors wanted to test a more close to real-life scenario of circadian rhythm disruption: they put wildtype mice on a HFD and exposed them to constant light conditions. After just three months, these mice, whose activity levels were acyclical during the forever-lasting light day, have triple the fat mass than their HFD-fed, but light/dark cycled littermates. This might be attributed to their decreased physical activity although they also tended to eat less food.
The implications of this study: the disruption of circadian rhythm leads to insulin resistance and significant body fat gain.

A mouse with an underlying condition feeding itself into obesity. Source
3. Leptin resistance
First, they found that PER deficient, as well as chronically jet-lagged wild-type mice, accumulate superfluous fat mass compared to wildtype mice kept in conventional conditions. Average food intakes and activity levels did not differ between these groups of mice.

Light/dark cycling scheme for inducing a chronic jet lag. Source
However, they found significant differences in the respiratory exchange ratio (RER, a breathing gas based parameter reflecting the state of the balance between glucose and fat fueled metabolism) and O2 consumption and CO2 production measured as volume flow. Shortly, the jet-lagged wild-type mice showed similarly abnormal values as the PER deficient mice. Although the authors do not provide a concise explanation for this finding, they conclude that their observed weight gain in the perturbed mice is due to their abnormal energy homeostasis.
In the second step, the researchers measured blood leptin levels which cycle throughout the day with a peak in the middle of the waking/activity phase. Chronically jet-lagged and PER deficient mice present with abnormally high and acyclical leptin levels along with flattened oscillations of the central molecular clock elements.
Leptin is produced by adipose tissue and the more adipose tissue there is, the higher leptin levels can go. Leptin production is controlled by another transcription factor called C/EBPalpha whose action is itself controlled by CLOCK/BMAL1 to yield an oscillating pattern of leptin transcription. In chronically jet-lagged mice this rhythmicity is abolished.
Lastly, leptin action in the ArcN is disrupted such as the appropriate response to leptin by signaling satiety to the animal is not carried out. This is characteristic of central leptin resistance.
The implications of this study: Circadian rhythm perturbation leads to a dysregulated energy homeostasis and the blunting of the circadian leptin secretion pattern along with a central leptin resistance. This misalignment results in fat mass gain.
4. Intermittent fasting to mitigate the deleterious effects of a disruptive diet
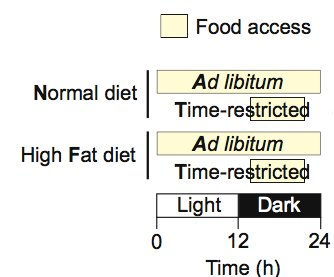
The feeding schemes for the 4 different groups of wildtype mice. Source
Let's first look at what happens to mice fed a HFD ad libitum for 4 months. They get incredibly fat despite eating the same amount of calories as the other groups. Molecularly, they show a persistent gluconeogenesis signal reflected by constantly elevated pCREB levels in the liver and a lack of pS6 activity during feeding, reflective of perturbed insulin activity. The oscillations of molecular clock components in the liver are blunted.
In accordance with the previously cited studies, the ad libitum HFD-fed mice show impaired glucose tolerance and elevated leptin and insulin levels, indicative of hormone-action resistance. Furthermore, these mice show an altered metabolite profile with elevated inflammatory fatty acids, decreased bile production, decreased anabolic sugar channeling and a decrease in the antioxidative reduced glutathione.
Lastly, these changes go hand in hand with inflammation of adipose tissue and a fatty liver disease (hepatosteatosis) with enlargement of the organ.
In summary, HFD ad libitum fed mice develop obesity and a metabolic disease.
All these detrimental effects of the “unhealthy” diet were almost completely ablated by restricting the feeding window to 8 hours/day during the waking period! Even mice fed normal chow experienced an improvement of metabolic parameters and inflammatory markers with time-restricted feeding.

A HFD ad libitum fed mouse versus a HFD fed mouse on a time-restricted feeding schedule Source
The implications of this study: The negative effects of a very poor diet in mice can be almost completely prevented by restoring the “natural” rhythmicity in feeding limited to a restricted period of the waking/activity phase of the circadian cycle.
Put mice prone to obesity on an intermittent fasting diet and they become lean and sexy!
Summary
It is only an induction, and it would be interesting to test this experimentally, but I postulate that time-restricted feeding would have, to some degree, ameliorated the negative effects of circadian disruption described in studies 2 and 3.
If you have any additional studies to complement this overview or any other remarks, please feel free to comment!
Stay tuned for part II covering available evidence for the benefits of intermittent fasting / time-restricted feeding in humans!

Source
~~~ So long, stay lean and healthy!~~~
Yours, @replichara
References
1. Ibanez C. The 2017 Nobel Prize in Physiology or Medicine - Advanced Information: Discoveries of Molecular Mechanisms Controlling the Circadian Rhythm. Nobelförsamlingen - Nobel Assembly at Karolinska Institutet. 2017. Nobel Media AB 2014.
2. Bass J. & Takahashi J.S. Circadian Integration of Metabolism and Energetics. Science, 2010 Dec 3;330(6009):1349-54.
3. Lamia K.A., Papp S.J., Yu R.T., Barish G.D., Uhlenhaut N.H., Jonker J.W., ... & Evans R.M. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature, 2011 Dec 14;480(7378):552-6.
4. Shi S.Q., Ansari T.S., McGuinness O.P., Wasserman D.H. & Johnson C.H. Circadian disruption leads to insulin resistance and obesity. Current Biology, 2013 Mar4;23(5):372-81.
5. Kettner N.M., Mayo S.A., Hua J., Lee C., Moore D.D. & Fu L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metabolism, 2015 Sep 1;22(3):448-59.
6. Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E.A., Gill S., ... & Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metabolism, 2012 Jun 6;15(6):848-60
Hi @replichara very interesting, i have been having problems with my corcadian rhythm, i have been working night shifts ever since i satrted my professional career, im also a night person ever since, but i think the western diet really is a killer, all those processed and dairy and meat are just a normal way of life. I am guilty as well, thanks for sharing this added info.
Thank you for reading and resteeming @errymil!
Yes, shift work, unfortunately, takes a toll on the body and research is figuring out how and why and what to do to make it less harmful.
The diet is definitely a strong modifying factor with processed high-carb foods probably being the culprits.
Hopefully, I can shed some light on that in the next post.
Do you feel you are more creative during night? :-)
Yes i feel more creative, i like the silent hrs as having 3 little kids at home is chaotic and messy most of the time hehe.
Very well done. I too have played around with intermittent fasting. I'm very much looking forward to parts 2 and 3 of this series. Upvoted and resteemed. Also, I'll try to snag an upvote from @originalworks for you.
The @OriginalWorks BETA V2 bot has upvoted(0.5%) and checked this post!
Some similarity seems to be present here:
https://en.wikipedia.org/wiki/2007_Nobel_Peace_Prize
This is an early BETA version. If you cited this source, then ignore this message! Reply if you feel this is an error.
Nah, I wasn't talking about peace, although it would be neat if IF would bring peace by rectifying faulty human behaviour.
Thanks!
It's fascinating how something that simple, as limiting the daily time window of eating, can have such significant effects.
Definitely worth an upvote and a resteem :]
Thanks!
This post has received a 7.72 % upvote from @booster thanks to: @irime.
Congratulations @replichara, this post is the eighth most rewarded post (based on pending payouts) in the last 12 hours written by a User account holder (accounts that hold between 0.1 and 1.0 Mega Vests). The total number of posts by User account holders during this period was 2024 and the total pending payments to posts in this category was $1820.13. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.