Hi Steemians!
The first chemists established a distinction between the compounds that constitute rocks and soils; the compounds that originate during the growth of plants and animals. Under this point of view the latter are formed by means of a vital force associated with the process of life its synthesis in the laboratory was impossible. According to this, they are called organic compounds, differentiating them from said mineral compounds, which are called inorganic compounds.
The range of organic compounds (carbon compounds) is really extensive, which is why this laboratory, which is a small introduction, will be based mainly on how these compounds are treated and their allotropic forms and essentially on making organic chemistry known from the carbon compound, its forms, react and alloys with and with respect to other elements in nature.
Source
Inorganic chemistry
The inorganic chemistry is the section of the world of the chemistry that is in charge of the study as far as structure, nomenclature, composition and chemical reactions in which are involved inorganic compounds, that is to say, compounds that within their molecules do not find bonds between carbons or hydrogens, in case such a union exists, it will be studied by organic chemistry. Mostly the compounds that are studied by inorganic chemistry are salts, acids, bases and oxides where carbon is not compromised, being divided in turn into metallic and non-metallic oxides; the inorganic compounds have less variety and quantity with respect to the organic ones.
Source
According to its layout and structure, inorganic compounds can be classified as binary and tertiary. Within the group of binary compounds is found: metal oxides, resulting from the conjugation between a metal and oxygen, according to which they are periodically observed in the environment they are known as basic oxides; On the other hand another element belonging to this group are anhydrides, this differs from the basic oxides because they result from a conjugation of oxygen with a non-metallic element, these are also known as acid oxides.
Another binary compound would be peroxide, this is the combination of two molecules of oxygen with a metal; likewise to this group belong the hydrides which are a product of the combination between hydrogen and a metal, they can be subclassified into volatile and non-volatile according to their capacity for combustion; As the last member of the binary compounds are the salts, these are products of the union between metal elements being highly volatile, it is also cataloged as a salt to the conjugation between metallic and non-metallic element, however these are not volatile.
On the other hand are the tertiary compounds within which can be mentioned, hydroxides are made by means of the conjugation of a hydroxide and a metal, likewise it is mentioned to the oxoacids that it is not more than the union between hydrogen, a compound non-metallic and oxygen; On the other hand, when a metal is conjugated with a non-metallic compound and oxygen, an oxisal is obtained, the last member of the tertiary compounds.
- Properties and configuration
Inorganic chemistry is a heterogeneous matter. The properties of the different chemical elements are not sufficiently variable so that broad generalizations can not be made. Therefore, the elements are usually studied by groups. Part of the properties of the elements depend on their electronic configuration. The elements belonging to the same group have equivalent configurations.
- Special groups
Some groups of elements have traditional names. For example, the lower elements of groups VIII and IB are called noble metals. This is because they are not attacked by almost any acid. They are relatively scarce elements in nature. They are ductile and have a great chemical resistance. In the same way, the elements of group VIIIA are known as noble gases. All of them are presented in a gaseous state at room temperature. In addition, its reactivity is very low. For this reason hardly any chemical compounds of noble gases are known.
Source
Organic chemistry
The Organic Chemistry is the branch of the Chemistry that studies the structure, behavior, properties and uses of the compounds that contain carbon, as much of natural origin as artificial. Carbon-containing compounds are called organic compounds. Therefore, organic chemistry studies organic compounds. Later we will see why it is called like that.
Source
This definition excludes some compounds such as carbon oxides, carbon salts and cyanides and derivatives, which by their characteristics belong to the field of inorganic chemistry. But these are just a few compounds against the thousands of compounds that organic chemistry studies.
Living beings are composed of organic compounds, therefore they are part of the study of organic chemistry, but beware, there are many organic compounds that are not present in living beings and that are also part of this branch of chemistry.
We could also say that organic chemistry is the one that studies molecules that contain carbon (C) and form carbon-carbon or carbon-hydrogen covalent bonds and other heteroatoms.
Organic compounds present a huge variety of properties and applications, among which we can mention: plastics, detergents, paints, explosives, pharmaceuticals, dyes, insecticides, perfumes, etc.
- The carbon atom
The symbol for the carbon atom is "C". It is a solid formed mainly by chains of carbon atoms. The carbon found on Earth was created about 5000 million years ago, during the formation period of the solar system, in which nuclear fusion chemistry prevailed, and it showed to be relatively stable. This allowed him to contribute an amount that represents 0.02% by weight of all the elements. Although this percentage seems low, carbon is the 12th most abundant element on our planet.
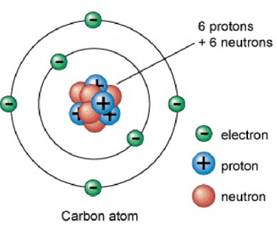
Source
The carbon belongs to group 14 of the periodic table, whose elements are: carbon (C), silicon (Si), germanium (Ge), tin (Sb) and lead (Pb). The first three are non-metals, and the last two are metals. All these elements share the catenation capacity, but none of them do it as easily as carbon.
In addition to concatenating, carbon can do so through multiple bonding, which means linking together through double and triple bonds. This last property is common to nitrogen and oxygen, but in such cases, cathenation is relatively rare.
Carbon atoms can be linked together in a variety of ways and in a number of atoms, impossible for any other element. They can form chains of thousands of atoms or rings of all sizes; These chains and rings may have ramifications. Other atoms are attached to the carbons of these chains and rings; mainly hydrogen, oxygen, fluorine, chlorine, bromine, iodine, nitrogen, sulfur, phosphorus. This particular characteristic is what allows so many carbon compounds to exist. The number of compounds containing carbon is several times greater than the number of substances that do not contain it.
- Hydrocarbons
Hydrocarbons are chemical substances coming from nature, which are formed by carbon and hydrogen.
These atoms are arranged in different forms, because their chains of carbon atoms can be open or closed and linear or branched, and can thus form various types of hydrocarbons, among which, the most significant are oil and natural gas.
These substances are originated in the deep layers of the earth, through millions of years, caused by the decomposition of plants and animals of remote times. And, this is why, they are the main compounds of organic chemistry.

Source
When the extraction of the hydrocarbon occurs in a liquid state, from a geological formation, it is called oil. On the other hand, the hydrocarbon that is naturally found in the gaseous state is called natural gas. The exploitation of these two elements, oil and natural gas, represents an essential industry for the economy, because it allows it to produce fossil fuels, as well as lubricants, plastics, among other products.
- Functional groups
The properties of carbon compounds depend on the arrangement of their chains and the types of atoms to which they are attached, that is, their structure.
A functional group is an atom or an array of atoms that always react in a certain way; In addition, it is the part of the molecule responsible for its chemical behavior since it gives it characteristic properties. Many organic compounds contain more than one functional group.
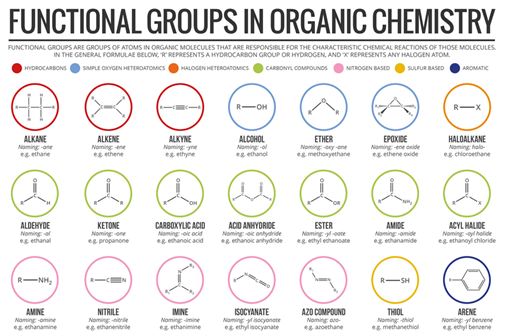
Source
- Alcohols, aldehydes and fatty acids
Alcohols are organic compounds that contain a hydroxyl group (-OH) as a functional group that determines the characteristic properties of this family. Variations in the structure of the alkyl group may affect the speed of certain reactions of the alcohol, and may have different applications. To name an alcohol the suffix -ol derived from the word alcohol is used. General formula R-OH
Aldehydes are compounds that result from the mild oxidation and dehydration of primary alcohols. The functional group of the aldehydes is the carbonyl as well as the ketone with the difference that in the aldehydes they go in a primary carbon, that is to say, of the ends.
Fatty acids are organic monoenoic acids, which are present in fats, rarely free, and almost always esterifying glycerol and eventually other alcohols. They are generally straight chain and have an even number of carbon atoms. The reason for this is that in the metabolism of eukaryotes, the fatty acid chains are synthesized and degraded by the addition or elimination of acetate units. However, there are exceptions, since fatty acids with an odd number of carbon atoms are found in the milk and fat of ruminants, from bacterial metabolism of the rumen, and also in some vegetable lipids, which are not commonly used for obtaining oils.
Fatty acids as such (free fatty acids) are rare in food, and are also generally a product of lipolytic alteration. However, they are fundamental constituents of the great majority of lipids, to the point that their presence is almost defining of this class of substances.
- Aromaticity
The phenomenon of aromaticity is one of the essential phenomena in organic chemistry. If sp3 hybridization leads to stereochemistry, sp2 hybridization allows the construction of flat molecules that can give rise to the phenomenon of aromaticity.
The term aromaticity encompasses several concepts without it being certain that all reflect a common reality. One of the reasons for this to occur is of a semantic nature: the word has not changed while the conception of the phenomenon has evolved with chemistry.
There are different definitions of the concept of aromaticity. In terms of molecular orbital theory, aromatic compounds are defined as fully conjugated monocyclic planar structures that have a particularly stable arrangement of occupied molecular orbitals, and whose system contains 4n + 2.
For more information visit the following links:
Thank you for the info!